Functionality of the utero-tubal unit
Dominique de Ziegler, Renato Fanchin, Jean-Marc Ayoubi, Carlo Bulletti
Department of Obstetrics and Gynecology, Reproductive Endocrinology and Infertility, Hôpital de Nyon, Switzerland
and Rimini Hospital, Rimini Italy
Address for correspondence:
D. de Ziegler, M. D,
Hôpital de Nyon,
1260 Nyon, Switzerland
Fax +(41-22) 994 6209
e-mail: ddeziegler@compuserve.com
1 Introduction
Uterine contractility of the non-pregnant uterus follows 3 characteristic patterns throughout the menstrual cycle: a) During the late follicular phase, i.e., at the peri-ovulatory time, uterine contractions (UC) frequency increases under the influence of E2. At this time in the cycle, UC are predominantly retrograde, playing a role in the rapid transport of sperm from the cervical area to the distal end of the tubes where fertilization takes place. b) During the luteal phase, i. e., after ovulation, an abrupt decrease in UC frequency characterizes the utero-relaxing effects of P c) During the luteo-follicular transition, i.e., during menses, antegrade contractions (fundus to cervix) predominate. Characteristically, all layers of the myometrium are involved in the contractile process involved in the forward emptying of uterine content (menstrual blood). At this time of the cycle, UCs are commonly perceived by women, sometimes causing (dysmenorrhea).
The methodology used for assessing uterine contractions (UC) evolved from invasive approaches, the recording of intra-uterine pressure (IUP), to non-invasive methods based on direct visualization of contractile events on ultrasound (UTZ) scans. UTZ based approaches provide validated data on UC frequency. UC direction however, can only be assessed by analyzing the displacement of substances (contrast markers) placed in the uterine cavity.
2 The Late Follicular Phase: retrograde transport of sperm toward the distal end of the Fallopian tubes.
Irrespective of the methodology used, all authors have unanimously described an increase in UC frequency at or near the time of ovulation (1-8). A consensus also exists for invoking E2 as the primary stimulant for the increase in UC frequency observed in the late follicular phase. Most publications including our own (1-3, 9, 10), concur at showing mean UC frequency of up to 4-6 UC/min at the time of ovulation. Characteristically however despite the relatively high UC frequency, these are notoriously painless. UTZ based data show that solely the sub-endometrial layers of the myometrium are involved in late follicular phase contractility (2, 4, 11, 12), often dubbed "sub-endometrial" contractions (4). The viewing of UTZ scans also led to describe late follicular phase UCs as "wavelike contractions" or "uterine peristaltsim" (3).
Late follicular phase UCs have been implicated in the rapid transport of sperm that reaches the pelvic cavity within minutes of intercourse (13, 14), well before it could get there animated by its own motion. Hence, contractility of the female tract (uterus and tubes) is seen as the primary mechanism assuring the rapid transport of sperm from the cervical area to the distal end of the tubes where fertilization takes place.
UTZ based studies have their limitation. Deducing UC direction from UTZ sequences (3, 7, 15) images has never been validated and is possibly misleading and/or erroneous (16). In order to study the true actual displacement of uterine content induced by uterine contractility, Leyendecker's team adapted (5, 8) an existing Tc 99-based hystero-salpingo-scintigraphy (HSS) technique (17). In an original work, these investigators used the Tc-99 based methodology for studying the functionality of the utero-tubal contractile unit rather than merely assessing tubal patency as provided by HSGs. With this methodology, Leyendecker et al. (5, 8, 18) showed that Tc-99 labeled macro albumin aggregates (MAA) placed in the vaginal fornix rapidly traveled toward the uterus and the Fallopian tubes. These authors claimed that retrograde transport toward the Fallopian tubes only occurred if a >16mm follicle was present and then, it was predominantly if not exclusively toward the tube facing the developing follicle or "ipsi-lateral" tube (5, 8).
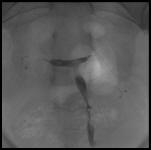
We developed a different approach aiming at assessing retrograde contractility in clinical conditions that mimic treatments commonly offered to women suffering from unexplained infertility (and therefore, at risk of dyskinetic alterations). For this, we used an X-ray based method (16), to study the retrograde transport during the late follicular phase. The actual procedure consisted in duplicating the conditions prevailing in intrauterine inseminations (IUI) (16). For this, we gently deposited with an embryo transfer catheter approximately 0.5 ml of an iodine-based contrast medium, (Isteropack) in the middle portion of the uterine cavity. Displacement of the contrast medium, retrograde toward one or both tubes or, antegrade toward the vagina, was followed on fluoroscopy, and illustrated on 1-5 successive X-rays (Fig. 1). Results were positive if retrograde transport was observed toward 1 or both tubes and intra-peritoneal spillage witnessed. They were negative if transport was toward the vagina and inconclusive, if contrast medium stagnated in the uterine cavity for >5min.
Using this novel X-ray based approach, we observed a prompt emptying of uterine content toward the Fallopian tubes and the pelvic cavity in 53.5% of women. On the contrary, all the contrast medium was rapidly expelled toward the vagina in 43.9% of women. In the remaining 2.6% of women, the contrast medium stagnated in the uterus for >5min. In 85.3% of women showing retrograde transport, this took place toward both tubes rather than solely toward the ipsi-lateral tube facing the developing follicle as described by Leyendecker’s team (5, 8). We postulated that the discrepancy with Leyendecker’s data reflects differences in sensitivity of the two methods used (16). While X-ray detection is exquisitely sensitive, that of Tc-99 by g-cameras is not. Hence, we postulate that the lateralization of transport detected by Leyendecker is relative rather than absolute, being only detected by the less sensitive method (Tc-99 MAA).
Using their Tc-99 based HSS approach, Leyendecker's group reported a characteristic disruption of the retrograde transport normally seen in the late follicular phase, in women suffering from endometriosis (5). Based on their findings, they claimed that endometriosis is associated with a state of hyperkinetic dyskinesia (5, 12, 18). We made concordant observations with our X-ray-based study of late follicular phase contractility (16). Hence, defective retrograde contractility and sperm transport likely represents a contributing factor in the infertility that mangles women suffering from mild to moderate infertility.
Different approaches have been contemplated for correcting uterine dyskinesia in the late follicular phase. Leyendecker's team studied the administration of an utero-contractant, oxytocin (18) when women lacked retrograde transport. No conclusive results exist on the efficacy of this treatment (18). In an earlier study (19), we tested the value of administrating synthetic prostaglandins (misoprostol 0.2mg) vaginally at the time of IUI on the assumption that "washed" sperm is deprived of most of its prostaglandins. This prospective randomized double blind study showed higher pregnancy rates in women supplemented with misoprostol as compared to controls (19). In the study, misoprostol was administered in custom-made glycerin base suppositories and tolerance was good (19). After study completion however, we administered misoprostol tablets vaginally instead of the custom made suppositories but encountered an unacceptable incidence of side effects (severe uterine cramping), forcing us to discontinue this practice (20).
3 The Luteal Phase: uterine quiescence provided by progesterone
From earlier studies based on analyzing intra uterine pressure (IUP), we know that the luteal phase is characterized by a prompt abatement of uterine contractility, rapidly leading to a state of relative uteroquiescence (1, 9, 10, 29). In an UTZ based study, we showed that UC frequency decreases from approximately 5 UC/min when the uterus is exposed to E2 only to <2.5 UC/min as early as 2-4 days after exposure to vaginal P (21). Similar UC values were encountered in the menstrual cycle by us (9, 10) and other teams (3, 4, 6).
Taken together, these data indicate that P confers relative utero-quiescence expressed by a decrease in UC frequency and a lack of expulsion of uterine content. The mechanism by which P exerts its utero-relaxing properties has not been totally elucidated yet. Some advocate a non-genomic mode action mediated either directly on cell membranes, or after local or distant metabolism into the 5-a reduced, 3-a hydroxilated metabolite of P, allopregnanolone (22). We recently reported evidence for local metabolism of P in the endometrial glands and stroma (23).
Using an UTZ based approach (Fig. 2), we showed that UC frequency remained elevated (at follicular phase values) at the time of ET in a large fraction of IVF patients (24). Contrasting with findings made in the luteal phase of the menstrual cycle by us (9, 10, 21) and others (1-3, 7), a large fraction of IVF patients had higher UC frequencies at the time of ET, at/or nearing 5 UC/min (24). This carries an ominous predicament, as we showed a strong inverse correlation between UC frequency at the time of ET and IVF outcome (Fig. 3) (25).
From these and other studies (26), we reckon that IVF patients resist the utero-quiescent effects of P, possibly because of higher E2 levels. On the day of ET, some women have persistently high UC frequency (24) despite higher P levels than in the menstrual cycle (10). The most practical recommendation for these women is to extend in vitro culture and delay transfers until the blastocyst stage (10).
4 The luteo-follicular phase transition (menses): expulsive contractions for a mini replica of labor.
During the luteo-follicular transition and early follicular phase, i. e., at the time of menses, UC are predominantly antegrade (1, 9) with displacement occurring from the fundus to the cervical end of the uterus (1, 9). This pattern of contractility is instrumental for the proper forward emptying of uterine content (menstrual blood) as well as hemostasis. Characteristically, the contractility pattern during menses represents a miniature replica of the expulsive forces of labor (1, 9). At this phase of the menstrual cycle, UCs can be (and typically, are) perceived by patients as cramps. On occasion, UCs can become frankly painful to the point of requiring specific medications and/or time off work, a condition known as dysmenorrhea. Studies based on IUP recordings and UTZ findings typically show lower UC frequencies at the time of menses at approximately 2.5/min than during the late follicular phase when peaks at 5 UC/min are reached (9). During the luteo-follicular transition, UC are characterized by high amplitudes with values up to 60mmHg (9). Resting tone is also elevated at approx. 40 mmHg, the highest value in the menstrual cycle. Studies assessing uterine contractility by electrophysiology have identified a trans-parietal involvement of all layers of the myometrium whereas, only the sub-endometrial layers are involved in late-follicular phase contractions.
The 2 primary alterations of uterine contractility at the time of menses are dysmenorrhea and endometriosis. Dysmenorrhea is a clinical condition characterized by painful, possibly invalidating uterine cramps occurring just prior to, or at the time of menses. A common disorder mainly affecting young women, dysmenorrhea can be invalidating for one or several days each month and cause numerous missed workdays. Uterine contractility at the time of menses in women suffering from dysmenorrhea is characterized by an elevation of resting IUP, UC amplitude, duration, and frequency (27). These characteristics of dysmenorrhea are only assessable by IUP recordings.
Endometriosis is a serious and potentially devastating disease of unknown origin that mainly affects gynecological and other organs/areas of the lower pelvic cavity. One of the 2 hypotheses put forth for explaining the development and growth of endometrial implants in various areas of the pelvic cavity incriminates retrograde bleeding, the so called "Sampson's retrograde menstruation theory" (28). According to this hypothesis, retrograde bleeding will disseminate endometrial fragments toward the pelvic cavity where they will implant and develop (28-30). Sampson's theory of retrograde menstruation has long been opposed to the alternate mechanism of "coelomic metaplasia" put forth for explaining endometriosis. Yet until now, no compelling evidence has ever led to the supremacy either one of these 2 theories. Today however, breaking away from a lingering opposition between these 2 concepts, new emerging views propose complementary rather than antagonizing roles for the 2 mechanisms. Hence, retrograde bleeding is seen as one of the phenomena (rather than the sole mechanism) that fuels endometriosis. In an UTZ based study neither confirmed by other approaches nor even reproduced to this date, Salamanca et al. (31) observed predominantly retrograde UCs at the time of menses in women with documented endometriosis. On the contrary, in the hands of these investigators, antegrade contractions predominated in unaffected controls. These findings quickly sparked the concept that endometriosis is intimately linked dyskinetic alterations of UC during the early follicular phase.
Inferences drawn from other forms of smooth muscle dyskinesia like notably, spastic bowel syndrome (32), led us (33) and others (5) postulate that the alterations of contractility at the heart of endometriosis probably are of the hyperkinetic type. The net results of these dyskinetic changes are variable degrees of impediment in the proper antegrade emptying of menstrual blood (29), which will exit through all opening possible (including retrograde). In this view, retrograde bleeding is one of the factors fueling the development of endometriotic implants through direct seeding with debris of endometrial tissue and activation of a chronic inflammatory reaction. The variable degree of inflammation generated by retrograde bleeding in the pelvic cavity and the resulting coelomic metaplasia will influence the degree of disease. Then, the very high hormonal concentration at the vicinity of the ovaries (through local diffusion and spillage of huge amounts at the time of follicular rupture) will favor the attachment of endometrial debris carried by retrograde bleeding in the pelvic cavity.
To clarify the issue of retrograde bleeding and pelvic seeding with endometrial fragments, we studied the amounts of endometrial debris present in the pelvic cavity just after menses (29). These studies showed that women suffering from endometriosis had more endometrial debris that displayed a stronger disposition to grow in culture than controls unaffected by endometriosis (29). Concordant findings were made by Leyendecker's team looking at the vagina-to-uterus transport of macro-albumin aggregates (MAA) labeled with Tc-99 and placed in the vaginal fornix (5, 8, 18). During the luteo-follicular transition, they found more extensive retrograde transport toward the uterus and tubes of Tc-99 MAA placed in the vaginal fornix in case of endometriosis (5).
5 Conclusion
Uterine contractility follows distinctive patterns during the various phases of the menstrual cycle. Alterations of uterine contractility are clinically important. During the late follicular phase, uterine contractility is instrumental in the rapid transport of sperm toward the distal end of the Fallopian tubes where fertilization takes place. Disruptions of contractility observed in women suffering from endometriosis impact of fertility as seen with even the mildest forms of this disease. During the luteo-follicular transition (menses), disruption of uterine contractility increases cramping (dysmenorrhea) or increases retrograde bleeding and peritoneal seeding of endometrial debris, a factor in the genesis of endometriosis.
6 References
Martinez-Gaudio M, Yoshida T, Bengtsson LP. Propagated and nonpropagated myometrial contractions in menstrual cycles. Am J Obstet Gynecol 1973;115:107-11.
De Vries K, Lyons EA, Ballard G, Levi CS, Lindsay DJ. Contractions of the inner third of the myometrium. Am J Obstet Gynecol 1990; 162:679-82.
Abramowicz JS, Archer DF. Uterine endometrial peristalsis-a transvaginal ultrasound study. Fertil Steril 1990; 54:451-4.
Lyons EA, Taylor PJ, Zheng XH, Ballard G, Levi CS, Kredenster JV. Characterization of subendometrial myometrial contractions throughout the menstrual cycle in normal fertile women. Fertil Steril 1991; 55:771-4.
Leyendecker G, Kunz G, Wildt L, Beil D, Deininger H. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Hum Reprod 1996;11:1542-51.
Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristaltis and hysterosalpingoscintigraphy. Hum Reprod 1996;11:627-32.
Ijland MM, Evers JLH, Dunselman GAJ, Volovics L, Hoogland HJ. Relation between endometrial wavelike activity and fecundability in spontaneous cycles. Fertil Steril 1997; 67:492-6.
Kunz G, Noe M, Herbetz M, Leyendecker G. Uterine peristalsis during the follicular phase of the menstrual cycle: effects of estrogen, antiestrogen and oxytocin. Human reprod Update 1998;4:647-54.
Bulletti C, de Ziegler D, Polli V, Diotallevi L, Del Ferro E, Flamigni C. Uterine contractility during the menstrual cycle. Hum Reprod. 2000;15:81-9.
Ayoubi JM, Brioschi PA, EpineyM, Fanchin R, Capana A, de Ziegler D. Instauration of Uterine Quiescence after Ovulation: Differences between Menstrual and IVF Cycles. Fertil Steril, Submitted for publication, 2002.
Noe M, Kunz G, Herbertz M, Mall G, Leyendecker G. The cyclic pattern of the immunocytochemical expression of oestrogen and progesterone receptors in human myometrial and endometrial layers: characterization of the endometrial-subendometrial unit. Human Reprod 1999;14:190-7.
Leyendecker G, Kunz G, Noe M, Herbertz M, Mall G. Endometriosis and disease of the archimetria. Human Reprod Update 1998;4:752-62.
Settlage DS, Motoshima M, Tredway DR. Sperm transport from the external cervical os to the fallopian tubes in woemn: a time and quantitation study. Fertil Steril 1973;24:655-61.
Moghissi KS. Human and bovine sperm migration. Fertil Steril 1968;19:118-22.
Chalubinski K, Deutinger J, Bernaschek G. Vaginosonography for recording of cycle-related myometrial contractions. Fertil Steril 1993;59:225-8.
de Ziegler D, Brioschi PA, Fanchin R, Bulletti C, Ditesheim PJ, Fonjallaz S, di Bernardo J. Retrograde transport of uterine content at mid cycle: the mock IUI model. Fertil Steril, Submitted for publication, 2002.
Lundberg S, Wramsby H, Bremmer S, Lundberg HJ, Asard PE. Radionuclide hysterosalpingography is not predictive in the diagnosis of infertility. Fertil Steril 1998;69:216-20.
Wildt L, Kissler S, Licht P, Becker W. Sperm transport in the human female genital tract and its modulation by hysterosalpingoscintigraphy, hysterotonography, electrohysterography and Doppler sonography. Human Reprod Update 1998;4:655-66.
Brown SE, Toner JP, Schnorr JA, Williams SC, Gibbons WE, de Ziegler D, Oehninger S. Vaginal misoprostol enhances intrauterine insemination. Hum Reprod. 2001;16:96-101.
Toner J, de Ziegler D, Brown S, Gibbons WE, Oehninger S, Schnorr JA, Williams SC. High rates of cramping with misoprostol administration for intrauterine insemination. Hum Reprod. 2001;16:1051.
Ayoubi JM, Fanchin R, Kaddouz D, Frydman R, de Ziegler D. Uterorelaxing effects of vaginal progesterone: comparison of two methodologies for assessing uterine contraction frequency on ultrasound scans. Fertil Steril. 2001;76:736-40.
Cabral R, Gutierrez M, Fernandez AI, Cantabrana B, Hidalgo A. Progesterone and pregnanolone derivatives relaxing effect on smooth muscle. Gen Pharmac 1994; 25:173-8.
C. Bulletti, D. De Ziegler, S. Boschi, M. Moreo, C. Flamigni. Uterine metabolism of progesterone (P4) after vaginal administration in an extracorporeal organ perfusion system. American Society of Reproductive Medicine 2001, Abs # S71
Fanchin R, Righini C, Olivennes F, Taylor S, de Ziegler D, Frydman R. Uterine contractions at the time of embryo transfer alter pregnancy rates after in-vitro fertilization. Hum Reprod. 1998;13:1968-74.
Fanchin R, Ayoubi JM, Olivennes F, Righini C, de Ziegler D, Frydman R. Hormonal influence on the uterine contractility during ovarian stimulation. Hum Reprod. 2000;15:90-100.
Fanchin R, Righini C, Schonauer LM, Olivennes F, Filho JS, Frydman R. Vaginal versus oral E(2) administration: effects on endometrial thickness, uterine perfusion, and contractility. Fertil Steril. 2001;76:994-8.
Strömberg P, Åkerlund M, Forsling MI, Granstrom F, Kindahl H. Vasopressin and prostaglandins in premenstrual pain and primary dysmenorrhea. Acta Obstet Gynecol Scand 1984; 63:533-8.
Sampson JA, Albany NY. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol 1927;14:422-69.
Bulletti C, de Ziegler D, Polli V, Del Ferro E, Palini S, Brioschi PA, Flamigni C. Uterine Contractility: Occurrence and Recurrence of Endometriosis. Fertil Steril, Accepted for publication, 2002.
Halme J, hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. J Am Coll Ob Gyn 1984;64:151-4.
Salamanca A, Beltran E. Subendometrial contractility in menstrual phase visualized by transvaginal sonography in patients with endometriosis. Fertil Steril 1995;64:193-5.
Maxwell PR, Mendall MA, Kumar D. Irritable bowel syndrome. Lancet 1997;350:1691-5.
Bulletti C, deZiegler D, Stefanetti M, Cincinelli E, Pelosi E, Flamigni C. Endometriosis: absence of recurrence in patients after endometrial ablation. Hum Reprod. 2001;16:2676-9.
7 Illustrations
7.1 Fig. 1: Retrograde transport in the late follicular phase assessed by X-ray contrast medium.
0.5 cc of X-ray contrast medium was deposited in the uterine cavity using a mock-IUI technique. This was followed by prompt expulsion of medium toward the Fallopian tubes and bilateral spillage in the pelvic cavity. A: Positive retrograde transport of uterine content after mock-IUI was observed in 53.5% of an infertile population of women whose tubes were patent. B: Negative retrograde transport. The contrast medium present in the uterine cavity was soon (<1min) totally expelled out toward the vagina in 43.9% of cases.
7.2 Fig 2: Assessment of UC frequency by ultrasounds (3-D method)
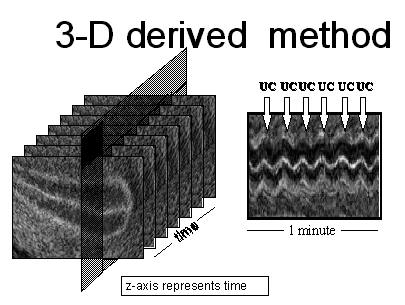
UC are assessed by direct visualization on ultrasound scans. An off-line 3-D system acquires a 3-D matrix in which the Z-axis is time because the ultrasound probe was not swept (kept still) during the acquisition period. With the 3-D matrix, electronically generated, time mode (TM) graphs can be obtained from any point of a transverse scan of the uterus. On TM graphs, UCs appear as vertical displacement of the myometrial-endometrial interface or "waves".
7.3 Fig. 3: UC at the time of ET and pregnancy rates
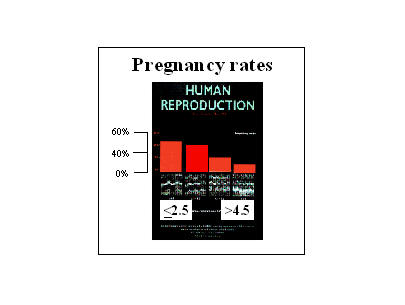
On the day of UC, a large fraction of women have elevated UC values. An inverse correlation exists between UC frequency on the day of ET and IVF outcome.