Postgraduate Training Course in Reproductive Health 2004
Phenotypic Features with p53 Alterations Related to HPV
and Prognostic Evaluation in Cervical Cancer
Dr. Xin Lu
Ob & Gyn Hospital at Fudan University, Shanghai
See also
![]() presentation
presentation
Abstract
Background: Cervical cancer is one of the most common tumors affecting
women worldwide. The multiple genetic alterations involved in the stepwise
development of this malignancy. There is evidence that human papillomavirus
(HPV) were found have a causal relationship with cervical cancer and its
precursors. The tumor suppressor gene p53 have been shown to be associated
with the prognosis for certain tumors. The interaction between HPV E6 protein
and p53 was identified in vitro studies.
Objectives: To evaluate the prevalence of p53 alterations related to HPV
infection, such as p53 mutation, LOH of p 53, p53 polymorphism; To evaluate
the prognostic significance of p53 alterations in cervical cancer.
Methods: Studies were identified by a MEDLINE search and all relevant articles
were retrieved from 1991 to March 2004. Some data from the World Health
Organization (WHO) and p53 database of the International Agency for Research
on Cancer (IARC) were also cited. All references not match the selective
criteria were excluded.
Results: The prevalence of p53 mutations is rare event in cervical cancer.
The correlation between p53 mutations and HPV or prognosis is controversy.
LOH of p53 is more common found in cervical cancer and related with the
prognosis of this disease. There is no significant correlation between p53
polymorphism and the development of cervical cancer.
Discussion: The significance of various p53 alterations with prognosis in
cervical cancer are discussed. The p53 mutations were found not common in
cervical cancer. LOH of p53 may contribute to the progression of this malignancy.
p53 mutations and p53 polymorphism failed to be an independent prognostic
factor in predicting the outcome of patients with cervical cancer. Further
epidemiological surveys should be undertaken in larger populations and in
different geographical regions.
Introduction
1. Introduction of cervical cancer
Cervical cancer is one of the most common and aggressive malignancy in female. Worldwide about 500 000 women acquire the disease annually and 75% are from developing countries (1,2). Cervical cancer has leading mortality rate in the world, every year around 300 000 women die of this disease. Although the etiology of cervical cancer has not been clear elucidated, but the causal relation between human papillomavirus (HPV) and the cervical cancer was well documented [3-6]. It was reported that around 95% of cervical cancers contain different types of HPV. To date, over 120 types of the HPV have been described (7). The World Health Organization (WHO), International Agency for Research on Cancer (IARC) has classified the HPV into three groups: “carcinogenic (HPV types 16 and 18); probably carcinogenic (HPV types 31 and 33) and possibly carcinogenic (other HPV types except 6 and 11) (6, 7). On the one hand, epidemiological studies had identified the major risk factors of cervical cancer. The population with HPV infection, multiple sex partners, smoking, oral contraceptives as well as family history have higher risk of cervical cancer (7-10). On the other hand, in addition to social-environmental and infectious factors, numerous molecular biologic studies have demonstrated that molecular genetic factors were also involved in the carcinogenesis of cervical cancer. These factors are cell cycle regulator genes, tumor suppressor genes, including p53 alteration (11-13).
2. Introduction of p53 gene
2.1 p53 gene structure and function
The first p53 gene mutation arising in a human cancer was described by Baker
(14). It is estimated that p53 mutaitons are the most frequent genetic events
in human cancers. During the past 25 years, p53 mutations are found in all
human cancers, account for more than 50% of the cases (15,16). The p53 gene
is located on chromosome 17p and is composed of 11 exons which encoding
for 393 amino acids. It is highly conserved in vertebrates. p53 gene contains
three domains: DNA binding domain, transactivation domain and oligomerization
domain (Fig. 1). The transcriptional domain at the N-terminus contains a
binding site for the product of MDM2 gene. The DNA binding domain is sequence-specific
and contains the binding sites for SV40 large T-antigen. The C-terminal
region contains a domain necessary for p53 oligomerization, one primary
and two secondary nuclear localization signal sequences, mediating non-specific
DNA binding. Wild type p53 is an important transcription factor and
coupling many functions. It was well-known that wild type p53 can acting
as a tumor suppressor, apoptosis inducer, and a protein able to arrest cell
cycle. p53 plays a critical role in the cell cycle regulation mechanisms
and cell proliferation control, and its inactivation is considered a key
event in human carcinogenesis (17).
2.2 p53 mutation in human cancer
p53 mutations are the main cause which contribute to its inactivation. For
most human cancers, p53 mutations are mis-sense mutations within the coding
sequence of the gene (Fig 1). About 90% of the mutations reported to be
clustered between exon 4 and 10 and are localized in DNA binding domain
of the p53 gene (15,16). The correlations between distinct mutants and functional
changes are well-established during past two decades. Mis-sense mutations
result in loss of p53 tumor suppress function by changing sequence-specific
transactivation activity (18). However, gain of oncogenic function by different
pathways which are controlled by this transcription factor was also seen
(19).
3. Introduction of p53 and HPV
Certain HPV serotypes have been associated with cervical cancers. Among HPV types, the most frequent one is HPV-16 (3-5). Previous studies have shown that in cervix, HPV-16 DNA is present in an episomal form in asymptomatic infections and benign lesions. While in malignant lesions it is frequently integrated into the host cell genome. The frequency of cervical cancer specimens that carry HPV-16 genome in integrated form ranges between 30-50% (20). The products of the E6 and E7 Open Reading Frames (ORFs) of HPV-16 genome are responsible of the immortalising effects on transformed epithelial cells. In vitro studies have demonstrated that the major transforming E6 and E7 activities include the targeted degradation of p53 and transcriptional induction of the cellular telomerase enzyme by E6 and the inactivation of the cellular retinoblastoma protein pRB by E7 (21, 22).
4. The prognostic factors of cervical cancer
The clinical and pathologic prognostic factors in cervical cancer are well defined, such as tumor histologic type, low grade differentiation, parametrial infiltration, lymph node metastases. A large study demonstrated that HPV 16 genotype may play an important role in the assessing progress of cervical cancer patients (23). In the literature, the alteration of mutated p53 leads to malignant phenotype, and the interaction between HPV and p53 pathways may contribute to cervical carcinogenesis. To better understand the role of p53 alterations related to HPV infection, and to predict the prognosis in cervical cancer, mutation, LOH of p 53 as well as p53 polymorphism will be reviewed.
Objectives
The objectives of this study are as follows:
- To analyse data about of p53 mutation in cervical cancer
- To investigate the correlation of p53 mutation and HPV infection in cervical carcinoma
- To evaluate whether p53 can be a prognostic factor in this malignancy
- To examine LOH of p53 and p53 polymorphism
Materials and Methods
Studies were identified by a MEDLINE search and all relevant articles from 1991 to March 2004 were retrieved. Searching the reference lists of retrieved full-text articles and abstracts, some relevant journals were obtained by hand searching. Data from the World Health Organization (WHO) and p53 database of the International Agency for Research on Cancer (IARC) were reviewed.
About p53 database
In 1997, a remarkable p53 database had been generated by IARC. The p53 mutation database contains all publications related on p53 gene alterations in human tumors cell lines and human cancers. It was compiled from the published literature and made available through electronic media. The database is now maintained at the IARC and is updated twice a year (24,25) and is available online. Today, there were over 18,585 mutations reported in the IARC database of human p53 tumor mutations (26,27). This database also provides an important information on the environmental factors and biological processes that are important causes of human gene aberrations (28).
The MEDLINE database was searched by using the following key words (MeSH):
Cervical cancer/carcinoma, HPV, p53 mutation, LOH , p53 polymorphism
References related to the following topics were reviewed: Etiological factors
for cervical cancer, the structure and function of p53 gene, interaction
between p53 gene and HPV E6 protein, the incidence of p53 mutation in cervical
cancer, correlation of p53 mutation with pathologic factors and prognosis,
LOH of p53 and p53 polymorphism in cervical cancer, methods commonly used
to detect p53 alterations.
So far, there are three methods used to detect mutations of p53 gene: immuno-histochemistry (IHC) to detect and localize the mutated protein expressed in cancer cells, Single-Strand Conformation Polymorphism (SSCP) to direct sequencing of the p53 gene and PCR based methods to analysis LOH of p53 which is located in 17p13 and polymorphism in p53. Each method has its own limitations in terms of specificity to detect p53 mutations. One important feature of mutated p53 protein is to increase stability and its accumulation in the nucleus of neoplastic cells. Positive immuno-staining is usually indicative of abnormalities of the p53 gene and its product, but it is highly dependent on the type of p53 mutation (29, 30).
Study Selection
The articles excluded were: 1). Technical papers without original data. 2). Letters and those that did not address cervical cancer or p53 mutation. 3). Those data only contain result from cell line study. 4). Paper that did not measure HPV and p53 mutation as the reference standard. The final sample for critical appraisal consisted of 103 studies. All articles were obtained from Medline, IARC p53 database as well as WHO library.
Results
1. Mutations of p53 in cervical cancer
1.1 Prevalence of p53 mutations and cervical
cancer
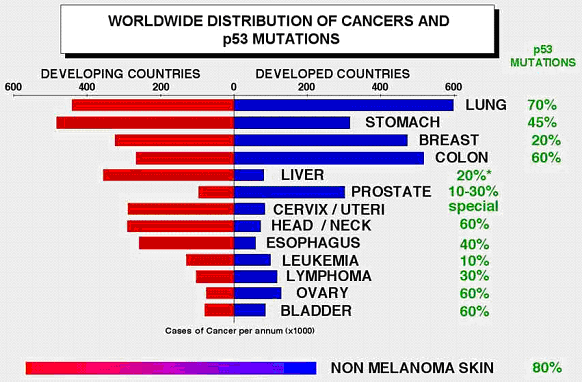
The worldwide distribution of human cancer countries is shown in Fig 2.
Compared to other type of cancers, p53 mutations were found not common in
cervical cancer (25).
Table 1. Prevalence of p53 mutation in cervical cancer based on gene sequencing. (SSCP : Single-Strand Conformation Polymorphism ; DDGE :Denaturing . Gradient Gel Electrophoresis ; CDGE : Constant Denaturant Gel Electrophoresis ; Yeast : Yeast Function Study).
| References | Country | Methods | Cases | Prevalence |
| Borresen AL et al. 1992 (31) | UK | CDGE | 92 | 2.17 |
| Fujita M et al. 1992 (32) | Japan | SSCP | 30 | 3.33 |
| Crook Tet al. 1992 (33) | UK | none | 28 | 10.71 |
| Paquette RL et al. 1993 (34) | USA | SSCP | 28 | 3.57 |
| Helland A et al.1993 (35) | Norway | CDGE | 92 | 2.17 |
| Kessis TD et al.1993 (36) | USA | SSCP | 29 | 3.45 |
| Busby-Earle RM et al.1994 (37) | UK | DGGE | 47 | 2.13 |
| Miwa K et al.1995 (38) | Japan | SSCP | 39 | 5.13 |
| Ikenberg H et al.1995 (39) | Germany | SSCP | 43 | 4.65 |
| Kim KH et al.1995 (40) | Korea | SSCP | 64 | 10.94 |
| Milde-Langosch K, et al. 1995(41) | Germany | SSCP | 51 | 7.8 |
| Kim JW et al. 1997 (42) | Korea | SSCP | 136 | 1.47 |
| Ngan HY et al. 1997 (43) | Hong-Kong | SSCP | 100 | 2.00 |
| Helland A et al. 1998 (44) | Norway | CDGE | 19 | 42.11 |
| Tenti P et al. 1998 (45) | Italy | DGGE | 74 | 13.51 |
| Gostout B et al. 1998 (46) | USA | none | 25 | 4.00 |
| Munirajan AK, 1998 (47) | India | SSCP | 43 | 9 |
| Limpaiboon T et al. 2000 (48) | Thailand | SSCP | 17 | 11.76 |
| Pinheiro NA et al. 2001 (49) | Brazil | SSCP | 122 | 3.28 |
| Harima Y et al. 2001(50) | Japan | SSCP | 65 | 10.77 |
| Denk C et al. 2001(51) | Germany | Yeast | 18 | 5.56 |
| Ishikawa H, et al. 2001(52) | Japan | SSCP | 52 | 26.9 |
According the data based on gene sequencing, from 19 studies, 13 studies showed the prevalence of p53 mutations less than 10% in cervical cancer (Table 1). Among them, only one study (43) showed high p53 mutation rate (42%). In two study, there is no significant difference between p53 mutations and histological type of cervical cancer(32,34).
1.2 Pattern of p53 mutations in cervical cancer
The complied data in the IARC p53 mutation database was used to analyze
the pattern of p53 mutations in cervical cancer. Fig 3 shows the pattern
and codon distribution of p53 mutations in cervical cancer. The codon distribution
in cervical cancer shows similar hotspots of mutations as in all other cancers.
The global analysis indicates that the p53 gene mutation pattern in cervical
cancer does not show features that significantly distinguish it from most
other human cancers.
1.3 Association of HPV infection and p53 mutations
in cervical cancer
Data from in vitro experiments showed that HPV E6 protein involved in p53
degradation. There are several groups have studied the correlation between
p53 mutations and HPV infection in cervical cancer patients (40, 43, 50-52).
The correlation between p53 mutation and HPV infection is controversy. Ishikawa
et al showed the p53 mutation was related with HPV infection (50). In contrast,
Helland reported that a significantly higher p53 mutation frequency was
found in HPV-negative cervical cancers (43). And the rest studies had shown
that there is no relationship between p53 mutations and HPV infection in
cervical cancer patients (40, 41, 47).
Table 2. Association between p53 mutation and HPV infection in cervical cancer (p53 data based on SSCP)
| References | Cases | Prevalence of p53 (%) |
Prevalence of HPV (%) |
P53 and HPV correlation |
| Ishikawa H, et al. 2001(52) | 52 | 26.9 | 76.9 | positive related |
| Helland A, et al. 1998 (43) | 365 | 42 | 76.5 | negative related |
| Munirajan AK, et al. 1998 (47) | 43 | 9 | 70 | not related |
| Milde-Langosch K, et al. 1995 (41) | 51 | 7.8 | 80.4 | not related |
| Kim KH, et al. 1995 (40) | 64 | 15.6 | 67.2 | not related |
2. The cervical cancer prognosis and p53 mutations
During past two decades, the major p53 alterations were found in cervical cancers and their precursors. These include p53 expression, mutations, LOH, as well as polymorphism in p53. This review analyzed the impact of these various p53 abnormalities on the cervical cancer patient prognosis. Overall, the IHC data showed that the correlation of p53 over expression with prognosis is discrepancy (53-66). In addition, because the p53 mutations are not common event in this malignancy, the evaluation of p53 mutations on the prognosis of cervical cancer remains to be defined (67).
2.1 p53 expression and cervical cancer prognosis based
on IHC studies
p53 gene mutation was found correlated with prognosis in a wide variety
of malignancies, especially in lung cancer and breast cancer (67). In this
review, although the prevalence of p53 prevalence based on IHC is much higher
than that based on gene sequencing, however the two groups (related and
unrelated prognosis) based on the IHC data showed no differences on prevalence
of p53 (46% vs 47.8%) (Table 3).
Table 3. p53 expression and prognosis in cervical cancer (IHC)
| Resource | Cases | Methods | P53 Prevalence (%) | Related with prognosis |
| Gitsch G, 1992 (53) | 43 | IHC | 46.5 | not related |
| Oka K, 1993 (54) | 192 | IHC | 25.5 | not related |
| Kainz C, 1995 (55) | 109 | IHC | 20.2 | not related |
| Benjamin I, 1996 (56) | 132 | IHC | 44 | not related |
| Kersemaekers AM, 1999 (57) | 136 | IHC | 32 | not related |
| Horn LC, 2001 (58) | 114 | IHC | 63.8 | not related |
| Ngan HY, 2001 (59) | 57 | IHC | 25.2 | not related |
| Haensgen G, 2001 (60) | 70 | IHC | 85.7 | not related |
| Total | 853 | 46.0% | ||
| Tsuda H, 1995 (61) | 26 | IHC | 46 | related |
| Bremer GC, 1995 (62) | 156 | IHC | 30.2 | related |
| Raju GC, 1996 (63) | 119 | IHC | 58 | related |
| Waggoner SE, 1996 (64) | 21 | IHC | 67 | related |
| Uchiyama M, 1997 (65) | 32 | IHC | 34 | related |
| Carrilho C, 2003 (66) | 45 | IHC | 50 | related |
| Total | 399 | 47.8% |
2.2 P53 expression and cervical cancer prognosis based
on SSCP studies
Because the p53 mutations are not common event in this malignancy and the
perspective study using gene sequencing limited the investigation, so there
are only two studies analysed the correlation between p53 mutations based
on SSCP and prognosis of cervical cancer patients (50,52). ISHIKAWA et al.
has studied 52 patients with squamous-cell carcinomas (SCC) who received
radiation therapy alone and investigated the effects of HPV infection, p53
status, and other parameters on clinical outcome by univariate analysis.
They found that the p53 mutation had a significant correlation with local
tumor recurrence, but no obvious correlation between HPV infections with
any clinical outcome for cervical cancer patients (50). Milde-Langosch K
et al. has reported 51 cervical cancers and 40 vulvar SCC for the presence
of HPV and mutant p53. In this study, p53 mutations were found in 7.8% in
cervical cancer and showed no relation with prognosis of the disease (52).
Table 4. p53 Mutation and Prognosis in Cervical Cancer (SSCP)
| References | Cases | Methods | p53 Prevalence (%) | p53 and prognosis |
| Ishikawa H, 2001 (50) | 52 | SSCP | 26.9 | related |
| Milde-Langosch K, 1995 (52) | 51 | SSCP | 7.8 | not related |
2.3 p53 mutation and radiotherapy (RT) in cervical
cancer
Cervical cancer is the most common cancer in women. Radiotherapy is
the first choice of treatment in those cases at late clinical stages. Even
though, the five-year survival rate remains low in those cases (68). It
is well known that the presence of mutant p53 is related to radio resistance
in a variety of tumor types (69,70). In cervical cancer, the results
are controversy. Five of eight reports demonstrated that the presence of
p53 was significant associated with the radio resistant of radiotherapy
(71-75) (Table 5). However, the rest three studies shown no relationship
between p53 and radio resistant of radiotherapy (76-78). We also reviewed
whether or not there is a correlation between p53 alteration and prognosis
related radiotherapy. Only two studies reported that p53 expression has
impact on prognosis in patients undergoing definitive radiotherapy for cervical
cancers (74, 75). They found that patients with immuno-histologically mutant
p53-negative tumors had a clear survival advantage over patients with immuno-histologically
mutant p53-positive carcinomas. However, another investigation found that
p53 expression did not influence survival in patients with primary cervical
cancers that were treated with radiotherapy (72).
Table 5. p53 alteration related with radiotherapy in cervical cancer (Prevalence of p53 based on IHC data; RT : radiotherapy ; ND: not detected)
| References | Cases | Prevalence of p53 (%) | p53 and Radiotherapy | p53 and prognosis |
| Niibe Y, et al.1999 (71) | 21 | 6.6 (before RT)
13.9 (after RT) |
related | ND |
| Oka K, et al. 2000 (72) | 202 | 52.1 | related | not related |
| Mukherjee G,et al. 2001(73) | 78 | 34 | related | ND |
| Jain D, et al.2003 (74) | 76 | 53.9 | related | related |
| Rajkumar T, et al.1998 (75) | 40 | 10 | related | related |
| Ebara T, et al.1996 (76) | 46 | 63 | not related | ND |
| Nakano T, et al.1998 (77) | 64 | 84.6 | not related | ND |
| Hove MG, et al. 1999 (78) | 22 | 11 recurrent 45.5 | not related | ND |
| 11 free 54.5 | not related | ND |
3. LOH of TP 53 in cervical cancer
3.1 Prevalence of LOH of p53 in cervical cancer
Cytogenetic analysis of cervical cancers has shown that chromosomes 1, 3, 11, and 17 are commonly abnormal (79). Chromosome 17 alterations are found in more cancers than those of any other chromosome, and frequently involve the p53 gene on 17p13. Most of studies showed that numerical abnormalities of chromosome 17 were found in cervical cancers (80-91) (Table 6). There is no correlation between LOH of p53 and HPV status in cervical cancers (87).
Table 6. Prevalence of LOH of p53 in cervical cancers (LOH: Loss of heterozygosity; ND: not detected)
| References | Cases | Prevalence of LOH | LOH and prognosis |
| Atkin NB, et al. 1990 (80) | 43 | 17 | ND |
| Kinoshita M, et al.1994 (81) | 11 | 36.4 | ND |
| Busby-Earle RM, et al.1994 (82) | 20 | 15 | ND |
| Mitra AB, et al. 1994 (83) | 17 | 41.2 | ND |
| Park SY et al. 1995 (84) | 26 | 40 | ND |
| Wistuba I et al. 1996 (85) | 12 | 50 | ND |
| Mullokandov MR, et al. 1996 (86) | 38 | 15 | ND |
| Kim JW et al. 1997 (87) | 55 | 5.5 | ND |
| Southern SA, et al. 1997 (88) | 25 | 36 | ND |
| Kersemaekers AMF, 1998 (89) | 64 | 38 | ND |
| Helland A et al. 2000 (90) | 79 | 18 | related |
| Harima Y et al. 2001 (91) | 65 | 33.8 | related |
3.2 LOH of p53 and prognosis in cervical cancer
The clinical course of the cervical cancer is highly complex, and genetic
factors underlying carcinogenesis are poorly understood. Loss on chromosomes
17p13.1 and p53 inactivation emerged as the disease progressed and were
closely associated with each other. There are some reports showing that
LOH of p53 related with the progression of certain tumor types (26). In
cervical cancer, two studies also found that the LOH of p53 related with
the clinical stages of tumors (90, 91) (Table 6).
4. p53 polymorphism in cervical cancer
4.1 Prevalence of p53 polymorphism in cervical cancer
Single nucleotide polymorphism (SNP) is a frequent form of single base pair
variations in genome, which occur once in every 1200-1500 base pairs by
the processes of deletion, addition, and substitution. These base pair variations
make each person's sequence unique. Thus the different susceptibility manifested
in each individual to a specific disease may also explained by genetic factors
in some cancers (92). It was known that the tumor suppressor gene p53 has
a SNP in codon 72. Polymorphism occurring in p53 codon 72 sequence causes
CGC to change to CCC at exon 4, changing the end product arginine to proline.
Table 7. Prevalence of p53 polymorphism in cervical cancer (Cxca: cervical cancer; Arg: arginine; Pro: proline; ND: not detected; NS: not significant)
| Resource | Populations | Cxca (%) | Control (%) | P value | Related with prognosis | Related with HPV | |
| Lee JE | Korea | Cases | 185 | 385 | |||
| 2004 (93) | Arg/Arg | 42.2 | 36.5 | NS | ND | ND | |
| Pro/Arg | 43.8 | 46.7 | |||||
| Pro/Pro | 14.1 | 16.8 | |||||
| Hernadi Z, | Hungary | Cases (39) | nodes (+) | nodes (-) | |||
| 2003 (94) | Arg/Arg | 54.5 | 67.9 | NS | NS | NS | |
| Pro/Arg | 45.5 | 21.4 | |||||
| Pro/Pro | 9.1 | 7.1 | |||||
| Pillai MR, | India | Cases | 232 | 189 | |||
| 2002 (95) | Arg/Arg | 20.2 | 18.5 | NS | ND | NS | |
| Pro/Arg | 48.4 | 51.3 | |||||
| Pro/Pro | 31.4 | 30.2 | |||||
| Nishikawa A, | Japan | Cases | 87 | CIN 28 | NS | NS | NS |
| 2000 (96) | Arg/Arg | 44.8 | 39.3 | ||||
| Pro/Arg | 42.5 | 35.7 | |||||
| Pro/Pro | 10.3 | 21.4 | |||||
| Klaes R, | Germany | Cases | 87 | 105 | NS | ND | NS |
| 1999 (97) | Arg/Arg | 54.6 | 55.7 | ||||
| Pro/Arg | 37.8 | 37.7 | |||||
| Pro/Pro | 7.6 | 6.6 | |||||
| Szarka K, | Hungary | Cases | 85 | 65 | |||
| 1999 (98) | Arg/Arg | 63 | 60 | NS | NS | ND | |
| Pro/Arg | 27 | 36 | |||||
| Pro/Pro | 10 | 4 |
In cervical cancer, most of studies showed that no relationship exists between p53 polymorphism and cervical cancer (93-98) (Table 7). However, there are studies showing that an over-expression of homozygous p53Arg in cervix cancer compared to heterozygous or homozygous p53Pro, suggesting that individuals homozygous for p53Arg genetically susceptible to cervical cancers (99-101). Recently, Koushik et al. summarized all data related to p53 polymorphism and cervical cancer in a meta-analysis (102). The meta-analysis has shown that there is no correlation between p53 polymorphism and cervical cancer.
4.2 P53 polymorphism and HPV prognosis in cervical
cancer
In this review, there are four studies showing that there is no significant
difference between p53 polymorphism and HPV of cervical cancer patients
(94-97). However, an India group have found that the frequencies of the
three p53 genotypes Arg/Arg, Arg/Pro and Pro/Pro in the HPV-positive tumour
samples were 0.34, 0.57 and 0.09 in comparison with frequencies of 0.18,
0.44 and 0.38 for HPV-negative tumours. There is a significant difference
in the allelic frequency of p53 Arg/Arg in high-risk HPV-infected cervical
carcinoma cases (0.34) and HPV-negative carcinomas (0.18) (103).
4.3 P53 polymorphism and prognosis in cervical cancer
A prospective cohort study was done to evaluation p53 codon 72 polymorphism
in predictive the outcome of cervical cancer with other factors (94). Among
39 patients with HPV-16 positive cervical cancer, there was no difference
between the lymph nodal metastases and p53 codon 72 polymorphism (Arg/Arg
54.5% vs 67.9%, Pro/Pro 0 vs 7.1%, Arg/Pro 45.5% vs 21.4%). The p53 codon
72 genotype did not influence the disease-free survival significantly (94).
Similarly, another two studies also showed that there is no significant
difference between p53 polymorphism and prognosis of cervical cancer patients
(96,98).
Discussion
The development of cervical cancer correlates with some risk factors (7-11). There is evidence showing that certain human papillomavirus types, such as HPV-16 and HPV-18 are the main cause of cervical cancer (3-5). HPV-16 and HPV-18 encode two major oncoproteins, E6 and E7. The E6 protein binds to the cellular tumour-suppressor protein p53 and directs its degradation through the ubiquitin pathway and the E7 protein binds to and inactivates the cellular tumour-suppressor protein Rb (21, 22). Although inactivation of these two tumour-suppressor proteins by HPV is probably important for tumour development. So far, no genetic factors have been conclusively identified that might an infected individual to develop cervical carcinoma. p53 mutations were found in many human tumours. However, according the data based on gene sequencing, from 21 studies, 14 studies showed the prevalence of p53 mutations less than 10% in cervical cancer (Table 1). Among them, only one study (43) showed high p53 mutation rate (42%). The result may be effected by selected samples. Because the p53 mutations are not common event in this malignancy, the perspective study using gene sequencing limited the investigation. It is controversy to use IHC to determine the prognosis. Although p53 immuno-histochemical analysis may be incomplete as a prognostic indicator, but the relationship to p53 mutation by sequence analysis is still not well defined. It has been shown that p53 over expression may result from either the short half-life of the wild type due to protein folding, over-production due to repair, or may be caused by technical factors. The evaluation of p53 over expression by immuno staining without molecular analysis may not give the exact informations. In this review, all studies on prognosis were based on IHC data. There is a need of large number of control study to compare differences between whether or not based on IHC and SSCP, further to confirm whether or not the p53 mutation related with prognosis as well as response to radio resistant of radiotherapy. If there is a correlation, It can be concluded that the assessment of the p53 status could aid in the selection of patients for different treatment strategies. The clinical course of the cervical cancer is highly complex, and genetic factors underlying carcinogenesis are poorly understood. Loss on chromosomes 17p13.1 and p53 inactivation emerged as the disease progressed and were closely associated with each other. There are some reports showing that LOH of p53 related with the progression of certain tumor types (26). In this review, only two studies found that the LOH of p53 related with the clinical stages of tumors (90, 91). In addition, the role of p53 polymorphism in cervical cancer remains to be elucidated (67). Although previous study had demonstrated that homozygous for p53Arg more likely to develop HPV-associated cervical cancer than individuals having one or more p53Pro alleles, suggesting that p53Arg may represent a risk factor for HPV-associated tumorgenesis (99). However, recently a meta-analysis on this issue was done by Koushik A et al. (102).They had shown that there is no evidence that p53 polymorphism is a risk factor in cervical cancer. In summary, the p53 mutations were found not common in cervical cancer. LOH of p53 may contribute to the progression of this malignancy. p53 polymorphism failed to be a risk factor in predicting the outcome of patients with cervical cancer. Understanding the behaviour of p53 alterations, and analysing it thoroughly for each patient could allow us to develop sound correlations between p53 status and patient outcome. Epidemiological surveys should be undertaken in larger populations and in different geographical regions to determine the role of various p53 aberrations in HPV-associated diseases and whether the p53 phenotype affects the persistence of HPV infection and the development of cervical cancer.
Conclusions
In this review, several phenotypic features with p53 alterations related to HPV are summarized and discussed. The tumor suppressor gene p53 mutations were found not common in cervical cancer compared with other cancer types. From the literatures, the correlation between p53 mutation and HPV infection is controversy. LOH of p53 has also been found in cervical cancers from several studies. LOH of p53 also related to the progression of this malignancy, but not related with HPV status. The p53 polymorphism failed to be a individual risk factor in predicting the outcome of patients with cervical cancer. In order to determine the role of various p53 aberrations in the development of cervical cancer, further epidemiological surveys should be undertaken in larger populations and in different geographical regions.
Acknowledgements
I do wish to thank Geneva Foundation for Medical Education and Research organizing this wonderful program.
Thanks IAMANEH for supporting me to join this course.
I also thanks all teachers for giving us wonderful lessons on methodology and advanced reproductive health.
Finally I am very grateful to my tutor Dr. Anis Feki, for helping me with this review correction.
References
- WHO/ 47. Press Release. Cervical Cancer: Experts Confirmed Virus A Major Cause, New Detection Technologies Available. 3 July, 1996.
- International Agency for Research on Cancer. IARC monographs on the evaluation of carcinogenic risks to humans; Human Papillomaviruses. Lyon, World Health Organization. 1995.
- zur Hausen H. Papillomavirus infections--a major cause of human cancers. Biochim Biophys Acta. 1996 Oct 9;1288(2):F55-78. Review [PubMed]
- Munger K, Howley, PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002 Nov;89(2):213-28. Review [PubMed]
- Cox T, Buck HW, Kinney W, Rubin MM. Human Papilloma Virus and Cervical Cancer. Clinical Proceedings. March 2001.
- WHO. Human Papilloma Virus (HPV). Summary of Data Reported and Evaluation. 1995.
- Bosch FX, Manos MM, Munoz N, Sherman M, Jansen AM, Peto J, Schiffman MH, Moreno V, Kurman R, Shah KV. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. International biological study on cervical cancer (IBSCC) Study Group. J Natl Cancer Inst. 1995 Jun 7;87(11):796-802. [PubMed]
- Ylitalo N, Sorensen P, Josefsson A, Frisch M, Sparen P, Ponten J, Gyllensten U, Melbye M, Adami HO. Smoking and oral contraceptives as risk factors for cervical carcinoma in situ. Int J Cancer. 1999 May 5;81(3):357-65. [PubMed]
- Schiffman MH, Brinton LA, Devessa SS, Fraumeni JF Jr. Cervical cancer. In: Schottenfeld D, Fraumeni JF Jr, editors. Cancer epidemiology and prevention. New York: Oxford University Press. 1996:1090-1116.
- Magnusson PK, Sparen P, Gyllensten UB. Genetic link to cervical tumours. Nature. 1999; 400:29-30.
- Wolf JK, Ramirez PT. The molecular biology of cervical cancer. Cancer Invest. 2001;19(6):621-9. Review. [PubMed]
- Tommasino M, Accardi R, Caldeira S, Dong W, Malanchi I, Smet A, Zehbe I. The role of TP53 in Cervical carcinogenesis. Hum Mutat. 2003 Mar;21(3):307-12. Review. [PubMed]
- Lazo PA. The molecular genetics of cervical carcinoma. Br J Cancer. 1999 Aug;80(12):2008-18. Review. [PubMed]
- Baker SJ, Fearon ER, Nigro JM, Hamilton SR, Preisinger AC, Jessup JM, vanTuinen P, Ledbetter DH, Barker DF, Nakamura Y, et al. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217-21. [PubMed]
- Soussi T, Dehouche K, Beroud C. p53 website and analysis of p53 gene mutations in human cancer: forging a link between epidemiology and carcinogenesis. Hum Mutat. 2000;15(1):105-13. [PubMed]
- Hollstein M, Sidransky D, Vogelstein B, Harris CC. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49-53. Review.[PubMed]
- Lane DP.Cancer. p53, guardian of the genome. Nature. 1992 Jul 2;358(6381):15-6. [PubMed]
- Kern SE, Kinzler KW, Bruskin A, Jarosz D, Friedman P, Prives C, Vogelstein B. Identification of p53 as a sequence-specific DNA-binding protein. Science. 1991 Jun 21;252(5013):1708-11. [PubMed]
- Hsiao M, Low J, Dorn E, Ku D, Pattengale P, Yeargin J, Haas M. Gain-of-function mutations of the p53 gene induce lymphohematopoietic metastatic potential and tissue invasiveness. Am J Pathol. 1994 Sep;145(3):702-14. [PubMed]
- Romanczuk H, Villa LL, Schlegel R, Howley PM. The viral transcriptional regulatory region upstream of the E6 and E7 genes is a major determinant of the differential immortalization activities of human papillomavirus types 16 and 18. J Virol. 1991 May;65(5):2739-44. [PubMed]
- Dyson N, Howley PM, Munger K, Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934-7. [PubMed]
- Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell. 1990 Dec 21;63(6):1129-36.[PubMed]
- Howley PM.Role of the human papillomaviruses in human cancer. Cancer Res. 1991 Sep 15;51(18 Suppl):5019s-5022s. Review. [ PubMed]
- Hernandez-Boussard T, Rodriguez-Tome P, Montesano R, Hainaut P. IARC p53 mutation database: a relational database to compile and analyze p53 mutations in human tumors and cell lines. International Agency for Research on Cancer. Hum Mutat. 1999;14(1):1-8. [PubMed]
- Hainaut P, Hernandez T, Robinson A, Rodriguez-Tome P, Flores T, Hollstein M, Harris CC, Montesano R. IARC Database of p53 gene mutations in human tumors and cell lines: updated compilation, revised formats and new visualisation tools. Nucleic Acids Res. 1998 Jan 1;26(1):205-13. [PubMed]
- Hollstein M, Hergenhahn M, Yang Q, Bartsch H, Wang ZQ, Hainaut P. New approaches to understanding p53 gene tumor mutation spectra. Mutat Res. 1999 Dec 17;431(2):199-209. Review. [PubMed]
- Hainaut P, Hollstein M, p53 and human cancer: the first ten thousand mutations. Adv Cancer Res. 2000;77:81-137. [PubMed]
- Beroud C and Soussi T. p53 gene mutation: software and database. Nucleic Acids Res. 1998 Jan 1;26(1):200-4. [PubMed]
- Dowell SP, Wilson PO, Deries NW, Lane DP, and Hall PA. Clinical utility of the immunocytochemical detection of p53 protein in cytological specimens. Cancer Res. 1994 Jun 1;54(11):2914-8. [PubMed]
- Casey G, Lopez ME, Ramos JC, Plummer SJ, Arboleda MJ, Shaughnessy M, Karlan B, Slamon DJ. DNA sequence analysis of exons 2 through 11 and immunohistochemical staining are required to detect all known p53 alterations in human malignancies. Oncogene. 1996 Nov 7;13(9):1971-81. [PubMed]
- Borresen AL, Helland A, Nesland J, Holm R, Trope C, Kaern J. Papillomaviruses, p53, and cervical cancer. Lancet. 1992 May 30;339(8805):1350-1. [PubMed]
- Fujita M, Inoue M, Tanizawa O, Iwamoto S, Enomoto T. Alterations of the p53 gene in human primary cervical carcinoma with and without human papillomavirus infection. Cancer Res. 1992 Oct 1;52(19):5323-8. [PubMed]
- Crook T, Wrede D, Tidy JA, Mason WP, Evans DJ, Vousden KH. Clonal p53 mutation in primary cervical cancer: association with human-papillomavirus-negative tumours. Lancet. 1992 May 2;339(8801):1070-3. [PubMed]
- Paquette RL, Lee YY, Wilczynski SP, Karmakar A, Kizaki M, Miller CW, Koeffler HP. Mutations of p53 and human papillomavirus infection in cervical carcinoma. Cancer. 1993 Aug 15;72(4):1272-80. [PubMed]
- Helland A, Holm R, Kristensen G, Kaern J, Karlsen F, Trope C, Nesland JM, Borresen AL. Genetic alterations of the TP53 gene, p53 protein expression and HPV infection in primary cervical carcinomas. J Pathol. 1993 Oct;171(2):105-14. [PubMed]
- Kessis TD, Slebos RJ, Han SM, Shah K, Bosch XF, Munoz N, Hedrick L, Cho KR. p53 gene mutations and MDM2 amplification are uncommon in primary carcinomas of the uterine cervix. Am J Pathol. 1993 Nov;143(5):1398-405. [PubMed]
- Busby-Earle RM, Steel CM, Williams AR, Cohen B, Bird CC. p53 mutations in cervical carcinogenesis--low frequency and lack of correlation with human papillomavirus status. Br J Cancer. 1994 Apr;69(4):732-7. [PubMed]
- Miwa K, Miyamoto S, Kato H, Imamura T, Nishida M, Yoshikawa Y, Nagata Y, Wake N. The role of p53 inactivation in human cervical cell carcinoma development. Br J Cancer. 1995 Feb;71(2):219-26. [PubMed]
- Ikenberg H, Matthay K, Schmitt B, Bauknecht T, Kiechle-Schwarz M, Goppinger A, Pfleiderer A. p53 mutation and MDM2 amplification are rare even in human papillomavirus-negative cervical carcinomas. Cancer. 1995 Jul 1;76(1):57-66. [PubMed]
- Kim KH, Kim YS. Role of human papillomavirus and p53 tumor suppressor gene in cervical carcinogenesis. Yonsei Med J. 1995; 36:412-425.
- Milde-Langosch K, Albrecht K, Joram S, Schlechte H, Giessing M, Loning T. Presence and persistence of HPV infection and p53 mutation in cancer of the cervix uteri and the vulva. Int J Cancer. 1995 Nov 27;63(5):639-45. [PubMed]
-
Kim JW, Cho YH, Lee CG, Kim
JH, Kim HK, Kim EJ, Han KT, Namkoong SE.
Human papillomavirus infection and TP53 gene mutation in primary cervical carcinoma. Acta Oncol. 1997;36(3):295-300. [PubMed] - Ngan HY, Tsao SW, Liu SS, Stanley M. Abnormal expression and mutation of p53 in cervical cancer--a study at protein, RNA and DNA levels. Genitourin Med. 1997 Feb;73(1):54-8. [PubMed]
-
Helland A, Karlsen F, Due
EU, Holm R, Kristensen G, Borresen-Dale A. MMutations in the TP53 gene
and protein expression of p53, MDM 2 and p21/WAF-1 in primary cervical
carcinomas with no or low human papillomavirus load.
Br J Cancer. 1998 Jul;78(1):69-72. [PubMed] - Tenti P, Pavanello S, Padovan L, Spinillo A, Vesentini N, Zappatore R, Migliora P, Zara C, Ranzani GN, Carnevali L. Analysis and clinical implications of p53 gene mutations and human papillomavirus type 16 and 18 infection in primary adenocarcinoma of the uterine cervix. Am J Pathol. 1998 Apr;152(4):1057-63. [PubMed]
- Gostout BS, Podratz KC, McGovern RM, Persing DH. Cervical cancer in older women: a molecular analysis of human papillomavirus types, HLA types, and p53 mutations. Am J Obstet Gynecol. 1998 Jul;179(1):56-61. [PubMed]
- Mullokandov MR, Kholodilov NG, Atkin NB. Genomic alterations in cervical carcinoma: losses of chromosome heterozygosity and human papilloma virus tumor status. Cancer Res. 1996 Jan 1;56(1):197-205. [PubMed]
- Limpaiboon T, Pooart J, Bhattarakosol P, Niruthisard S, Chantratita W, Lulitanond V. pp53 status and human papillomavirus infection in Thai women with cervical carcinoma. Southeast Asian J Trop Med Public Health. 2000 Mar;31(1):66-71. [PubMed]
- Pinheiro NA, Villa LL.Low frequency of p53 mutations in cervical carcinomas among Brazilian women. Braz J Med Biol Res. 2001 Jun;34(6):727-33. [PubMed]
- Harima Y, Sawada S, Nagata K, Sougawa M, Ohnishi T. Chromosome 6p21.2, 18q21.2 and human papilloma virus (HPV) DNA can predict prognosis of cervical cancer after radiotherapy. Int J Cancer. 2001 Oct 20;96(5):286-96. [PubMed]
-
Denk C, Butz K, Schneider
A, Durst M, Hoppe-Seyler F. p53 mutations are rare events in recurrent
cervical cancer.
J Mol Med. 2001 Jun;79(5-6):283-8. [PubMed] - Ishikawa H, Mitsuhashi N, Sakurai H, Maebayashi K, Niibe H. The effects of p53 status and human papillomavirus infection on the clinical outcome of patients with stage IIIB cervical carcinoma treated with radiation therapy alone. Cancer. 2001 Jan 1;91(1):80-9. [PubMed]
- Gitsch G, Kainz C, Joura E, Breitenecker G. Mutant p53 product in patients with stage III cervical cancer. Anticancer Res. 1992 Nov-Dec;12(6B):2241-2. [PubMed]
- Oka K, Nakano T, Arai T. p53CM1 expression is not associated with prognosis in uterine cervical carcinoma. Cancer. 1993 Jul 1;72(1):160-4. [PubMed]
- Kainz C, Kohlberger P, Gitsch G, Sliutz G, Breitenecker G, Reinthaller A. Mutant p53 in patients with invasive cervical cancer stages IB to IIB. Gynecol Oncol. 1995 May;57(2):212-4. [PubMed]
- Benjamin I, Saigo P, Finstad C, Takahashi H, Federici M, Rubin SC, Boyd J. Expression and mutational analysis of P53 in stage IB and IIA cervical cancers. Am J Obstet Gynecol. 1996 Nov;175(5):1266-71. [PubMed]
- Kersemaekers AM, Fleuren GJ, Kenter GG, Van den Broek LJ, Uljee SM, Hermans J, Van de Vijver MJ. Oncogene alterations in carcinomas of the uterine cervix: overexpression of the epidermal growth factor receptor is associated with poor prognosis. Clin Cancer Res. 1999 Mar;5(3):577-86. [PubMed]
- Horn LC, Fischer U, Hanel C, Kuhn H, Raptis G, Bilek K. p53 in surgically treated and pathologically staged cervical cancer: correlation with local tumor progression, but not with lymphatic spread. Pathol Res Pract. 2001;197(9):605-9. [PubMed]
- Ngan HY, Cheung AN, Liu SS, Cheng DK, Ng TY, Wong LC. Abnormal expression of pan-ras, c-myc and tp53 in squamous cell carcinoma of cervix: correlation with HPV and prognosis. Oncol Rep. 2001 May-Jun;8(3):557-61. [PubMed]
- Haensgen G, Krause U, Becker A, Stadler P, Lautenschlaeger C, Wohlrab W, Rath FW, Molls M, Dunst J. Tumor hypoxia, p53, and prognosis in cervical cancers. Int J Radiat Oncol Biol Phys. 2001 Jul 15;50(4):865-72. [PubMed]
-
Tsuda H, Jiko K, Tsugane
S, Yajima M, Yamada T, Tanemura K, Tsunematsu R, Ohmi K, Sonoda T, Hirohashi
S. Prognostic value of p53 protein accumulation in cancer cell nuclei
in adenocarcinoma of the uterine cervix.
Jpn J Cancer Res. 1995 Nov;86(11):1049-53. [PubMed] - Bremer GL, Tieboschb AT, van der Putten HW, de Haan J, Arends JW. p53 tumor suppressor gene protein expression in cervical cancer: relationship to prognosis. Eur J Obstet Gynecol Reprod Biol. 1995 Nov;63(1):55-9. . [PubMed]
- Raju GC, Teh M, Wee A. An immunohistochemical study of p53 protein in cervical intraepithelial neoplasia and squamous cell carcinoma. Pathology. 1996 Jan;28(1):17-19. [PubMed]
- Waggoner SE, Anderson SM, Luce MC, Takahashi H, Boyd J. p53 protein expression and gene analysis in clear cell adenocarcinoma of the vagina and cervix. Gynecol Oncol. 1996 Mar;60(3):339-44. [PubMed]
- Uchiyama M, Iwasaka T, Matsuo N, Hachisuga T, Mori M, Sugimori H. Correlation between human papillomavirus positivity and p53 gene overexpression in adenocarcinoma of the uterine cervix. Gynecol Oncol. 1997 Apr;65(1):23-9. [PubMed]
- Carrilho C, Gouveia P, Cantel M, Alberto M, Buane L, David L. Characterization of human papillomavirus infection, P53 and Ki-67 expression in cervix cancer of Mozambican women. Pathol Res Pract. 2003;199(5):303-11. [PubMed]
- Soussi T, Beroud C. Assessing TP53 status in human tumours to evaluate clinical outcome. Nat Rev Cancer. 2001 Dec;1(3):233-40. [PubMed]
- Marcial VA & Marcial LY. Radiation therapy of cervical cancer. New developments. Cancer. 1993 Feb 15;71(4 Suppl):1438-45. [PubMed]
- Prives C & Hall PA. The p53 pathway. J Pathol. 1999 Jan;187(1):112-26. [PubMed]
- Bristow RG, Benchimol S and Hill RP. The p53 gene as a modifier of intrinsic radiosensitivity: implications for radiotherapy. Radiother Oncol. 1996 Sep;40(3):197-223. [PubMed]
- Niibe Y, Nakano T, Ohno T, Tsujii H, Oka K. Relationship between p21/WAF-1/CIP-1 and apoptosis in cervical cancer during radiation therapy. Int J Radiat Oncol Biol Phys. 1999 May 1;44(2):297-303. [PubMed]
- Oka K, Suzuki Y, Nakano T. Expression of p27 and p53 in cervical squamous cell carcinoma patients treated with radiotherapy alone: radiotherapeutic effect and prognosis. Cancer. 2000 Jun 15;88(12):2766-73. [PubMed]
- Mukherjee G, Freeman A, Moore R, Kumaraswamy, Devi KU, Morris LS, Coleman N, Dilworth S, Prabhakaran PS, Stanley MA. Biologic factors and response to radiotherapy in carcinoma of the cervix. Int J Gynecol Cancer. 2001 May-Jun;11(3):187-93. [PubMed]
- Jain D, Srinivasan R, Patel FD, Kumari Gupta S. Evaluation of p53 and Bcl-2 expression as prognostic markers in invasive cervical carcinoma stage IIb/III patients treated by radiotherapy. Gynecol Oncol. 2003 Jan;88(1):22-8. [PubMed]
- Rajkumar T, Rajan S, Baruah RK, Majhi U, Selvaluxmi G, Vasanthan A. PPrognostic significance of Bcl-2 and p53 protein expression in stage IIB and IIIB squamous cell carcinoma of the cervix. Eur J Gynaecol Oncol. 1998;19(6):556-60. [PubMed]
- Ebara T, Mitsuhashi N, Saito Y, Sakurai H, Hasegawa M, Takahashi M, Takahashi T, Hayakawa K, Niibe H. Prognostic significance of immunohistochemically detected p53 protein expression in stage IIIB squamous cell carcinoma of the uterine cervix treated with radiation therapy alone. Gynecol Oncol. 1996 Nov;63(2):216-8. [PubMed]
- Nakano T, Oka K, Ishikawa A, Morita S. IImmunohistochemical prediction of radiation response and local control in radiation therapy for cervical cancer. Cancer Detect Prev. 1998;22(2):120-8.[PubMed]
- Hove MG, Dinh TV, Hannigan EV, Lucci JA 3rd, Chopra V, Smith ER, To T. Oncogene expression and microvessel count in recurrent and nonrecurrent stage Ib squamous cell carcinoma of the cervix. J Reprod Med. 1999 Jun;44(6):493-6. [PubMed]
- Atkin NB. Cytogenetics of carcinoma of the cervix uteri: a review. Cancer Genet Cytogenet. 1997 May;95(1):33-9. Review. [PubMed]
- Atkin NB, Baker MC, Fox MF. Chromosome changes in 43 carcinomas of the cervix uteri. Cancer Genet Cytogenet. 1990 Feb;44(2):229-41. [PubMed]
- Kinoshita M, Shin S, Hirao T, Aono T. A quantitative approach to the loss of heterozygosity in p53 allele on detection of human cervical cancer using a BioImaging Analyzer. Asia Oceania J Obstet Gynaecol. 1994 Mar;20(1):87-92. [PubMed]
- Busby-Earle RM, Steel CM, Williams AR, Cohen B, Bird CC. p53 mutations in cervical carcinogenesis--low frequency and lack of correlation with human papillomavirus status. Br J Cancer. 1994 Apr;69(4):732-7. [PubMed]
- Mitra AB, Murty VV, Li RG, Pratap M, Luthra UK, Chaganti RS . Allelotype analysis of cervical carcinoma. Cancer Res. 1994 Aug 15;54(16):4481-7. [PubMed]
- Park SY, Kang YS, Kim BG, Lee SH, Lee ED, Lee KH, Park KB, Lee JH. Loss of heterozygosity on the short arm of chromosome 17 in uterine cervical carcinomas. Cancer Genet Cytogenet. 1995 Jan;79(1):74-8. [PubMed]
- Yamamoto Y, Virmani AK, Wistuba II, McIntire D, Vuitch F, Albores-Saavedra J, Gazdar AF. Loss of heterozygosity and microsatellite alterations in p53 and RB genes in adenoid cystic carcinoma of the salivary glands. Hum Pathol. 1996 Nov;27(11):1204-10. [PubMed]
- Mullokandov MR, Kholodilov NG, Atkin NB, Burk RD, Johnson AB, Klinger HP. Genomic alterations in cervical carcinoma: losses of chromosome heterozygosity and human papilloma virus tumor status. Cancer Res 1996; 56:197–205. [PubMed]
- Kim JW, Lee CG, Han SM, Kim KS, Kim JO, Lee JM, Kim IK, Namkoong SE. Loss of heterozygosity of the retinoblastoma and p53 genes in primary cervical carcinomas with human papillomavirus infection. Gynecol Oncol. 1997 Nov;67(2):215-21. [PubMed]
- Southern SA, Herrington CS. Interphase karyotypic analysis of chromosomes 11, 17 and X in invasive squamous-cell carcinoma of the cervix: morphological correlation with HPV infection. Int J Cancer. 1997 Mar 4;70(5):502-7. [PubMed]
- Kersemaekers AM, Hermans J, Fleuren GJ, van de Vijver MJ. Loss of heterozygosity for defined regions on chromosomes 3, 11 and 17 in carcinomas of the uterine cervix. Br J Cancer. 1998;77(2):192-200. [PubMed]
- Helland A, Kraggerud SM, Kristensen GB, Holm R, Abeler VM, Huebner K, Borresen-Dale AL, Lothe RA. Primary cervical carcinomas show 2 common regions of deletion at 3P, 1 within the FHIT gene: evaluation of allelic imbalance at FHIT, RB1 and TP53 in relation to survival. Int J Cancer. 2000 Oct 15;88(2):217-22. [PubMed]
- Harima Y, Sawada S, Nagata K, Sougawa M, Ohnishi T. Chromosome 6p21.2, 18q21.2 and human papilloma virus (HPV) DNA can predict prognosis of cervical cancer after radiotherapy. Int J Cancer. 2001 Oct 20;96(5):286-96. [PubMed]
- Schork NJ, Fallin D, Lanchbury JS. Single nucleotide polymorphisms and the future of genetic epidemiology. Clin Genet. 2000 Oct;58(4):250-64. [PubMed]
- Lee JE, Lee SJ, Namkoong SE, Um SJ, Sull JW, Jee SH, You YK, Park JS. GGene-gene and gene-environmental interactions of p53, p21, and IRF-1 polymorphisms in Korean women with cervix cancer. Int J Gynecol Cancer. 2004 Jan-Feb;14(1):118-25. [PubMed]
- Harnadi Z, Szarka K, Sapy T, Krasznai Z, Veress G, Poka R. The prognostic significance of HPV-16 genome status of the lymph nodes, the integration status and p53 genotype in HPV-16 positive cervical cancer: a long term follow up. BJOG. 2003;110:205-9.
- Pillai MR, Sreevidya S, Pollock BH, Jayaprakash PG, Herman B. Polymorphism at codon 72 of p53, human papillomavirus, and cervical cancer in South India. J Cancer Res Clin Oncol. 2002 Nov;128(11):627-31. Epub 2002 Oct 09. [PubMed]
- Nishikawa A, Fujimoto T, Akutagawa N, Iwasaki M, Takeuchi M, Fujinaga K, Kudo R. pp53 Polymorphism (codon-72) has no correlation with the development and the clinical features of cervical cancer. Int J Gynecol Cancer. 2000 Sep;10(5):402-407. [PubMed]
- Klaes R, Ridder R, Schaefer U, Benner A, von Knebel Doeberitz M. No evidence of p53 allele-specific predisposition in human papillomavirus-associated cervical cancer. J Mol Med. 1999 Feb;77(2):299-302.[PubMed]
- Szarka K, Veress G, Juhasz A, Konya J, Sapy T, Soos G, Hernadi Z, Gergely L. Integration status of virus DNA and p53 codon 72 polymorphism in human papillomavirus type 16 positive cervical cancers. Anticancer Res. 2000 May-Jun;20(3B):2161-7. [PubMed]
- Storey A, Thomas M, Kalita A. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature. 1998 May 21;393(6682):229-34. [PubMed]
- Rosenthal AN, Ryan A, Hopster D, Jacobs IJ. p53 codon 72 polymorphism in vulval cancer and vulval intraepithelial neoplasia. Br J Cancer. 2000 Nov;83(10):1287-90. [PubMed]
- Andersson S, Rylander E, Strand A, Sallstrom J, Wilander E. The significance of p53 codon 72 polymorphism for the development of cervical adenocarcinomas. Br J Cancer. 2001 Oct 19;85(8):1153-6. [PubMed]
- Koushik A, Platt RW, Franco EL. p53 codon 72 polymorphism and cervical neoplasia: a meta-analysis review. Cancer Epidemiol Biomarkers Prev. 2004 Jan;13(1):11-22. [PubMed]
- Nagpal JK, Sahni S, Das BR. P53 codon 72 polymorphism and susceptibility to development of human papilloma virus-associated cervical cancer in Indian women. Eur J Clin Invest. 2002 Dec;32(12):943-8. [PubMed]
Figure 1:
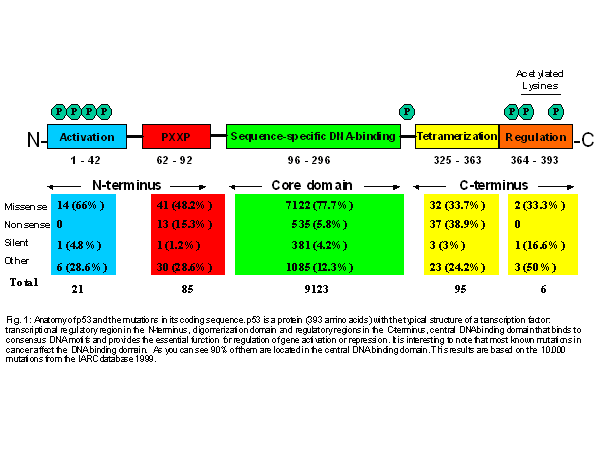
Fig 2. Worldwide distribution of cancers and p53 mutations.