Diaa M. El-Mowafi - Zagagig University, Egypt
Vaginal Dopamine Agonists: Biochemical and Clinical Responses - A New Trial
Diaa El-Mowafi(1)*, Mamdouh Youssef(2) and Mona A. Hassan(3
Obstetrics and Gynecology(1), Medical Biochemistry Departments(2)
Benha Faculty of Medicine - Zagazig University
and Pharmacology department (3)- Ain Shams Faculty of Medicine, Egypt
* To whom correspondance should be addressed : Diaa El-Mowafi, MD, 4 Ghazza st., El-Hossania, El-Mansoura 35111, Egypt. Fax + 2 050 332771
ABSTRACT
Thirty five hyperprolactinemic women were included in this study. They were attending Benha University Hospitals with different clinical manifestations such as primary and secondary infertility, secondary amenorrhea, oligomenorrhea, pre-menstrual tension syndrome and hirsutism. Eighteen patients had clinically manifested galactorrhea. Sixteen of our patients stopped oral dopamine agonist therapy since at least two months because of its side effects. Our patients were divided into two groups. Each woman in the first group was given one tablet (2.5 mg) of bromocriptine at night by vaginal route for three months , while each woman in the second group was given one tablet (0.2 mg) of lysuride each night by the same route and for the same duration. Serum prolactin was assayed for each case before , two weeks and three months after, starting therapy. A highly significant decline in serum prolactin levels (P < 0.0005) was achieved after two weeks of therapy on both vaginal bromocriptine (53.9% improvement) & vaginal lysuride (53.8% improvement). After three months, thirty patients reached a normoprolactinemic level while five patients, three on bromocriptine and two on lysuride, did not respond to the dose given and doubling the dose was not effective. Also, we found no significant difference between both drugs as they had the same effect on serum prolactin. All the clinical manifestations were relieved or improved including galactorrhea, but adjuvant therapy was needed in eleven patients with infertility, given adjuvant ovulatory drugs, three patients with hirsutism and one patient with secondary amenorrhea. All patients who discontinued the oral therapy because of its side effects did not report any complaint , while on vaginal route the biochemical and clinical response promotes the use of vaginal route as an alternative to the oral one for dopamine agonists therapy.
INTRODUCTION
Hyperprolactinia is a disorder which may be represented clinically by one or more of the following: galactorrhea, infertility, oligomenorrhea, pre-menstrual tension syndrome (PTS), amenorrhea, luteal phase defect and hirsutism. Bromocriptine, a dopamine agonist was the drug of choice for treatment of hyperprolactinemia and pituitary microadenomas(1). Because this therapy needs to be continued for several years in some cases, patient compliance is important in order to avoid discontinuation of the drug. The major reasons for discontinuation are the side effects namely; nausea, vomiting, postural hypotension, dizziness and headache occurring in more than half of patients (2).
Lysuride was developed later on and assumed to have less side effects although it was not free of it(3). Many drugs, including steroids and antibiotics are readily absorbed through the vaginal epithelium into the circulation. In contrast to oral administration, the vaginal administration of the drugs, results in slower absorption and a more constant level of the drug in the circulation and also avoids an initial high concentration of the drug in the gastrointestinal tract and liver(4).
This work aims to evaluate the clinical and biochemical response to the two dopamine agonists, bromocriptine and lysuride, as they are given by a new modality which is the vaginal route instead of the usual oral route they are available for.
SUBJECTS, MATERIALS and METHODS
Subjects
Thirty five hyperprolactinemic women attending Benha University Hospitals with different clinical presentations : Fourteen were complaining of secondary infertility, nine with oligomenorrhea, 4 with pre-menstrual tension syndrome, 3 with secondary amenorrhea, 3 with hirsutism, and 2 with primary infertility.
Eighteen of these cases presented with galactorrhea. All women were neither pregnant nor lactating during the study , not receiving any drug currently and had no any other complaint.
Sixteen of our patients have, stopped oral bromocriptine or lysuride therapy because of their side effects since a period not less than two months. Ages of patients range between 21 and 36 years, their weight ranged 52 - 89 Kg., and their gravidity ranges 0 and 3.
Drugs
- Bromocriptine (Parlodel,Sandoz) 2.5 mg / tablet.
- Lysuride ( Dopargine , Schering) 0.2 mg / tablet.
Methods
CT scan was done to all patients presenting with initial prolactin level higher than 100 ng /ml.
Three ml of venous blood samples were taken before , two weeks and 3 months after initiation of therapy. Sera were stored at -20 °C till the time of assay. The samples were taken from 9-11 AM after 30 min of rest with no coitus the day before. The patients were instructed to record any clinical manifestations during therapy.
Serum levels of prolactin were analysed by radioimmunoassay method using the HRRLK-PR Kit according to Cowden et al.(5).
All results were statistically analysed using student «t » test (Fisher and Yates, 1964) , and were tabulated in the following tables and figures.
RESULTS
Clinical results
As regard the clinical presentations, the three patients with hirsutism needed adjuvant therapy to control hirsutism inspite of the decline in prolactin level in two of them.
From the fourteen patients presenting with secondary infertility, three patients got pregnant three months on vaginal bromocriptine and two patients got pregnant on vaginal lysuride. Inspite of resuming ovulation as detected by basal body temperature chart (BBT), in seven of the remaining 9 patients on vaginal dopamine agonists only, those patients had to be given adjuvant therapy m the form of clomiphene or human monopausal gonadotrophin/human chorionic gonadotrophin (hMG/hCG).
The two patients presenting with primary infertility had to be given adjuvant therapy in spite of resuming the normoprolactinemic level and ovulation (by BBT) after three months from initiation of therapy.
Only one patient from the three who was presenting with secondary amenorrhea had to receive hMG therapy to get her menses back again.
All patients presenting with galactorrhea had been completely relieved and all other clinically manifested presentations had been improved.
Only one patient on vaginal bromocriptine therapy had slight nausea early in the course of treatment that resolved spontaneously. The sixteen patients who had side effects on oral therapy did not report any effect on vaginal therapy.
Biochemical results
Table 1: represents prolactin (ng/ml) in hyperprolactinemic women before and after starting therapy with vaginal bromoctiptine.
Table 2: represents prolactin (ng/ml) in hyperprolactinemic women before and after starting therapy with vaginal lysuride.
Table 3: represents mean values ± S.D of serum prolactin (ng/ml) in hyperprolactmemic women before and after starting therapy with both drugs.
By using bromocriptine vaginally, we found a significant fall in serum prolactin (P < 0.0005) from (71.3 ± 23.1) ng/ml to (32.9 ± 11.6) after two weeks of therapy i.e. 53.8% improvement and further significant fall (P < 0.0005) to become 10.6 ± 3.2 ng/ml after three months of therapy.
By using lysuride vaginally, we found a significant fall in serum prolactin (P < 0.0005) from (72.7 + 23.7) ng/ml to (33.6 + 15.4) ng/ml after 2 weeks of therapy i.e. 53.7% improvement and further significant fall (P < 0.0005) to become (12.0 ± 5.7) ng/ml after 3 months of therapy.
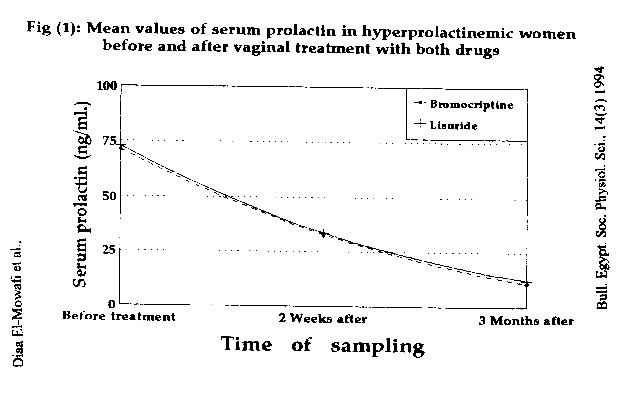
Figure (1): represents serum prolactin levels in hyperprolactinemic women before and after therapy with both vaginal bromocriptine and lysuride.
Five patients,from the thirty five whom were admitted into the study (three on bromocriptine: and two on lysuride) did not respond to the vaginal dose, given and doubling the dose was not effective. They had been excluded and refereed to neurosurgical evaluation.
By using the, student "t" test to statistically analyse the results obtained by both drugs regarding the degree of improvment, we found no significant difference between both drugs denoting that they had the same effect and efficiency.
DISCUSSION
Bromocriptine and lysuride had been manufactured to be given by oral (commonly used) or parenteral (rarely used) routes only (7). Discontinuation of the treatment due to its side effects particularly on gastrointestinal tract (2) had encouraged us to carry out this new trial for vaginal administration of the oral tablet.
Our study showed a significant fall (P < 0.0005) in the mean value of serum prolactin level from (71.3 + 23.1) ng/ml to (32.9 ± 11.6) ng/ml after two weeks i.e. about 53.9% improvement and to the normal level (10.6 ± 3.2) ng/ml after three months on vaginal bromocriptine (table 1, table 3 & figure 1). These results agree with that of Ginsburg and his fellows(4) who reported a fall in serum prolactin of 46.1 % 8 hours after a single, (2.5 mg) vaginal dose and a fall of 51.5% after two weeks.
Also, the mean value of serum prolactin levels showed a significant fall (P < 0.0005) from (72.7 ± 23.7) ng/ml to (33.6 + 15.4) ng/ml after two weeks i.e. about 53.7% improvement and to the, normal level (12.0 ±5.7) ng/ml after three months on vaginal lisuride (table 2, table 3 & figure 1).
No previous studies on lysuride given vaginally could be found.
Our results on vaginal dopamine agonists coincide with those found on oral therapy in doses ranges between 2-4 tablets bromocriptine, and 1.5-3 tablets of lysuride. Soto-Albers et al.(8) reported a fall of 56% in serum prolactin level two weeks after oral bromocriptine and a fall to normal values two months later. Also, De Pablo et al.(9) reported a decline of 59% in serum prolactin one week after oral bromocriptine and a fall to normal level three months later. While in oral lysuride therapy, Bouloux et al.(10) reported a decline of 48% after 10 days and a fall to normal levels 50 days later.
On evaluating the efficiency of vaginal dopamine agonists clinically, we found that all patients presents with galactorrhea had been completely relieved and all other clinically manifested presentations had been improved. Ginsburg et al.(4) had reported similar results.
Patients presenting with secondary infertility and got pregnant only after three months in our study represent 35.7% which is somewhat less than that recorded by Diedrich (11), who gave oral bromocriptine for 13 hyperprolactinemic anovulating patients , seven of them (53.8%) got pregnant on bromocriptine alone although he did not mention the duration of therapy after winch they got pregnant. Three of his patients got pregnant on bromocriptine and clomiphene and another three on bromocriptine plus hMG/hCG.
Scholz and Horowski (12) reported a pregnancy rate of 42.4% after 3 months of oral lysuride in an average dose 0.2 - 0.4 mg/day.
In the present study, no significant difference was found between both drugs winch had effect and efficiency on serum prolactin.
In conclusion, vaginal dopamine agonists (bromocriptine and lysuride) are well tolerated compared with oral therapy. We recommended the vaginal route for these drugs as it gives almost the same biochemical and clinical response of oral route with the advantage of lowering the dose and the costs and avoiding the gastrointestinal side effects.
REFERENCES
- Sheamm R. (1983): Inappropriate hyperprolactinemia and secondary amenorrhea. In: Recent Adv. Obstet. Gynecol., vol. 3 pp 256 - 265.
- Biggs J. and Edwards V. (1989): First-dose effects of bromocriptine in hyperprolactinemic infertility. Australian and New Zealand Joumal of Obstet. and Gynecol., 21.: 111 - 112.
- Vinturini P., Horowski R., Maganza C. and Morana S. (1991): Effects of lysuride and bromocriptine on inhibition of lactation and on serum prolactin levels: Comparative double blind study. European Journal of Obstet. and Gynecol. and Reproductive Biology., 11 :395 - 400.
- Ginsburg J., Hardiman P. and Thomas M. (1992): Vaginal bromocriptine: Clinical and biochemical effects. Gynecol. Endocrinol., 6: 119 -126.
- Cowden E.A., Ratchfce J.G., and Thomas J.A. (1979): Tests of prolactin secretion in diagnosis of prolactinomas. Lancet 1 : 1155.
- Fisher R. A and Yates F. (1964) : Tests of significance based on the normal distribution. In Statistical tables for biological agricultural and Medical Research. Oliver and Boyd, Edinburgh, London.
- Lengyel A., Vicira J., Mussio W. and Imamura P. (1993): Long acting injectable bromocriptine in the chronic treatment of prolactin secreting macroadenomas. Fertil. Steril., 59 : 980 - 987.
- Soto-Albers C., Daly D., Walten C., Ying Y. and Riddick D. (1985): Titrating the dose of bromocriptine when treating hyperprolactinemic women. J. Clin. Endocrinol. Metab., 69: 99.
- De Pablo F., Eastman R., Roth J. and Gordon P. (1991): Plasma prolactin in acromegally before and after treatment. J. Clin Endocrinol Metab., 93: 344 - 352.
- Bouloux P., Besser G. and Grossman A. (1987): Clinical evaluation of lysuride in the management of hyperprolactinemia. Lancet 1 : 612 - 615.
- Diedrich K. (1988): Pregnancies after treatment with bromocriptine. Obstet. Gynecol. 72 :520 - 562.
- Scholz A. and Horowski R. (1986): Proceeding of the 12th world congress on fertility and sterility, Singapore . Eds. Eng-Soon Teoh, Parthenon Publishing Group UK :159 - 162.
Table 1: Serum prolactin (ng/ml) in hyperprolactinemic women before and after starting therapy with vaginal lysuride
| Case No | Time of sampling | ||||
| Before treatment | 2 weeks after | Degree of improvement | 3 months after | Degree of improvement | |
| 1 | 62 | 33 | -29 | 16 | -46 |
| 2 | 80 | 40 | -40 | 15 | -65 |
| 3 | 57 | 20 | -37 | 12 | -45 |
| 4 | 58 | 26 | -32 | 11 | -47 |
| 5 | 73 | 30 | -43 | 17 | -56 |
| 6 | 60 | 28 | -32 | 10 | -50 |
| 7 | 48 | 18 | -30 | 9 | -39 |
| 8 | 66 | 29 | -37 | 8 | -58 |
| 9 | 92 | 48 | -44 | 8 | -44 |
| 10 | 120 | 50 | -70 | 12 | -108 |
| 11 | 48 | 23 | -25 | 11 | -37 |
| 12 | 124 | 60 | -64 | 12 | -112 |
| 13 | 74 | 34 | -40 | 10 | -64 |
| 14 | 74 | 38 | -36 | 8 | -66 |
| 15 | 56 | 25 | -31 | 6 | -50 |
| 16 | 50 | 25 | -25 | 6 | -44 |
| X | 71.3 | 32.9 | -38.4 | 10.6 | -58.1 |
| 5.0 | 23.1 | 11.6 | 12.58 | 3.2 | 22.09 |
| n. | 16 | 16 | 16 | 16 | 16 |
| S.E | 5.77 | 2.9 | 3.14 | 0.8 | 5.52 |
| Paired "t" | 12.22 | 10.5 | |||
Table 2: Serum prolactin (ng/ml) in hyperprolactinemic women before and after starting therapy with vaginal lysuride
| Case No | Time of sampling | ||||
| Before treatment | Two weeks after | Degree of improvement | Three months after | Degree of improvement | |
| 17 | 50 | 20 | -30 | 10 | -40 |
| 18 | 52 | 22 | -30 | 10 | -42 |
| 19 | 77 | 26 | -51 | 18 | -59 |
| 20 | 64 | 30 | -34 | 15 | -49 |
| 21 | 90 | 49 | -41 | 17 | -73 |
| 22 | 89 | 52 | -37 | 9 | -80 |
| 23 | 84 | 42 | -42 | 13 | -71 |
| 24 | 55 | 26 | -29 | 20 | -35 |
| 25 | 46 | 20 | -26 | 3 | -43 |
| 26 | 42 | 18 | -24 | 5 | -14 |
| 27 | 66 | 17 | -49 | 10 | -56 |
| 28 | 107 | 60 | -47 | 20 | -87 |
| 29 | 122 | 58 | -64 | 20 | -102 |
| 30 | 75 | 31 | -44 | 8 | -67 |
| X | 72.7 | 33.6 | -39.1 | 12.0 | -58.42 |
| 5.0 | 23.7 | 15.4 | 11.28 | 5.7 | 23.35 |
| n. | 14 | 14 | 14 | 141 | 14 |
| S.E | 6.33 | 4.11 | 3.01 | 1.52 | 6.24 |
| Paired "t" | 12.99 | 9.36 | |||
Table 3: Serum prolactin (ng/ml) in hyperprolactinemic women before and after starting therapy with both drugs Mean values ± S.D.
| Drug used | Time of sampling | ||
| Before treatment | Two weeks after treatment | Three months after treatment | |
| Bromocriptine | 71.3 ± 23.1 | 32.9 ± 11.6* | 10.6 ± 3.2 |
| Lysuride | 72.7 ± 23.7 | 33.6 ± 15.4* | 12.0 ± 5.7* |
*very highly significant P<0.0005