Traitement de la DMLA par les Sérocytol® (médicaments immunobiologiques)
The use of tissue antisera (Serocytol®) in age-related macular degeneration
Martine Mauget-Faÿsse§,1*, Michel Sickenberg1,2*, Michel Mpandi3, Thi Weber§,3
1 Centre Ophthalmologique Rabelais, 12-14 rue Rabelais 69003 Lyon, France
2 M.Sickenberg, Av. d'Ouchy 14, 1006 Lausanne, Switzerland
3 Serolab, Lausanne, Switzerland
*These authors contributed equally to this work
§Corresponding author
E-mail addresses:
MMF: centrerabelais@wanadoo.fr
MS: michel.sickenberg@bluewin.ch
MM: michel.mpandi@serolab.ch
TW: weber@serolab.ch
How to cite this article:
Mauget-Faÿsse M, Sickenberg M, Mpandi M, Weber T. The use of tissue antisera (Serocytol®) in age-related macular degeneration. Geneva Foundation for Medical Education and Research. 11 Aug. 2006.
Available from:
https://www.gfmer.ch/Presentations_En/Serocytol_age-related_macular_degeneration.htm
Abstract
Background
A retrospective study was carried out in 42 outpatients with age-related macular degeneration to assess the effectiveness and tolerance of tissue antisera “Œil” and tissue antisera “Neurovasculaire”.
Methods
A group of patients were prescribed, in addition to their regular treatment, with eye tissue antisera (Serocytol® “Œil”) twice a week, and nerves, vessels, connective, skin tissues antisera (Serocytol®
“Neurovasculaire”) once a week. The control group was only taking regular treatment. Standard protocol refraction, visual acuity testing (EDTRS charts), contrast sensitivity testing (Pelli-Robson test), ophthalmic examinations, color photographs and fluorescein angiographies were used to measure the evolution of the patients in both groups.
Results
Forty-two (42) age-related macular degeneration patients were included: 20 in the tissue antisera treated group (group 1): 4 male, 16 female, mean age 70.33, mean follow-up 4 years; and 22 in the untreated group (group 2): 6 male, 16 female, mean age 72.97, mean follow-up 3.5 years.
In group 1, 10 had an atrophic form of age-related macular degeneration, and 10 an exsudative form; in group 2, 12 were atrophic, and 10 exsudative. At inclusion, mean VA (visual acuity) was 0.53 in group 1, and 0.65 in group 2. At the end of follow-up: mean VA in group 1 and group 2 was 0.39. Contrast sensitivity testing at inclusion was available only for group 1: 1.25. At the end of the study: contrast sensitivity was measured at 1.06 in group 1 and 1.02 in group 2. General anatomic evolution was favorable in 14 patients in group 1 and in 5 patients in group 2 and worsen in 6 patients of group 1 and 17 patients of group 2. No adverse ocular or systemic harmful effect has been observed.
Conclusions
These results suggest a long-term good tolerance of equine tissue antisera “Œil” and “Neurovasculaire”. It seems also that this therapeutic approach has a beneficial effect on VA (visual acuity) and on age-related macular degeneration anatomic evolution. Clinical and basic scientific studies will help to clarify the action by which this therapy may provide some benefits.
Keywords: age-related macular degeneration, vision, equine, horse, tissue antisera, eye tissue, Serocytol, Serocytol Œil, nerve tissue, vessel tissue, connective tissue, skin tissue, Serocytol Neurovasculaire, suppository, biologic treatment, immunoglobulins, immunomodulation.
Background
Age-related macular degeneration (AMD) and particularly the exsudative form, which is a major complication of AMD disease, remains a leading cause of blindness in Western countries [1]. Etiological research suggests that AMD is a complex disease, caused by the actions and interactions of multiple genes and environmental factors.
While a variety of new therapies against choroidal new-vessels (CNV) are available or under evaluation, there are no proven therapies for treating efficiently the underlying disease itself, with the exception of high dose of vitamin supplements in a sub-group of AMD patients [2]. The natural evolution of AMD will still lead to progressive loss of central visual acuity and/or unpredictable neovascularization occurrences.
Drusen, which are the hallmark of the disease, are pathological deposits that form between retinal pigment epithelium (RPE) and Bruch’s membrane. Hageman et al [3] have provided a new hypothesis of the origin and evolution of these drusen in trying to identify specific molecular pathways associated with drusen biogenesis. Significantly, these studies have revealed that proteins associated with inflammation and immune-mediated processes are prevalent among drusen-associated constituents. These data have also lead to the observations that dendritic cells, potent antigen-presenting cells, are intimately associated with drusen development and that complement activation is a key pathway that is active both within drusen and along the RPE-choroid interface. This hypothesis invokes the potential for a direct role of cell- and immune-mediated processes in drusen biogenesis and could explain the beneficial role of an immunotherapeutical approach in this blinding pathology.
Furthermore, to confirm this hypothesis of Hageman, gene discovery provided clues to cause of age-related macular degeneration.
Recent acquisitions of knowledge in this disease have identified a major risk variant within the complement factor H gene (CFH) [4-6]. The complement system is a biochemical cascade of the immune system. Factor H (HF1), the major inhibitor of the alternative complement pathway, has been shown accumulate within drusen and is synthesized by the retinal pigmented epithelium [7].
Anti-tissue immunotherapy has been used empirically over more than 40 years. Its benefits have been reported in the treatment of a variety of inflammatory or degenerative diseases such as osteoarthrosis, fibromyalgia, chronic bladder infections and chronic gastritis [8,
9, 10, 11]. The rationale of the treatment is based in the use of specific incriminated tissue antisera (for example: bladder mucosa for chronic bladder infections) in order to obtain mostly an immunoregulation with clinical benefit some weeks or some months later.
The local inflammatory and immune-mediated abnormalities have been strongly suggested as a causative factor in AMD [3,
12, 13]. Therefore based on these observations, we decided to investigate the effects of tissue antisera “Œil” and “Neurovasculaire” in different eye diseases, particularly in AMD.
Methods
Patients
A group of patients with long standing AMD progression were clinically evaluated between 1994 and 2000 and were retrospectively analyzed in 2003. The treatment group (group 1) was prescribed in addition to their regular systemic treatment tissue antisera “Œil” twice a week, and tissue antisera “Neurovasculaire” once a week for 2 periods of 3 months a year. A particular attention was given to anamnestic presence of high blood pressure and/or the use of potentially photosensitizing drugs such as thiazidic diuretics, non-steroidal anti-inflammatory drugs, and so on [14,
15]. The control group (group 2) was only taking their regular systemic treatment.
Both studies groups were matched for sex, age, follow-up duration and were concomitantly observed in a single outpatient ophthalmic centre.
Inclusion criteria for both groups were: typical signs for AMD such as drusen and retinal pigment epithelium (RPE) alterations with proven progression signs such as geographic atrophy of the RPE and/or progression to any forms of exsudative AMD in one eye. The clinical evaluation at baseline and follow-up was always done on one eye, mostly the best functional one.
Standard protocol refraction, best corrected visual acuity (VA) testing with an EDTRS chart [16], contrast sensitivity testing with a Pelli-Robson chart [17], standard ophthalmic examinations, color photographs and fluorescein angiographies were used to evaluate the evolution of the patients in both groups.
The clinical end-points were both functional: changes in best corrected VA, comparison of contrast sensitivity at end of follow-up, and morphological: clinical evolution of the RPE atrophy.
Both groups were proposed for treatment, but group 2 patients denied the treatment for reasons such as absence of reimbursement by third party payers, way of drug administration (suppositories), and lack of confidence in this biologic treatment.
Tissue antisera (Serocytol®)
Tissue antisera are immunobiological products made by Serolab (Lausanne, Switzerland) and prepared with serum of horse immunized with a particular homogenized tissue or organ from pig. Suppositories used in this study contain 0.33 ml (20 mg of proteins) of equine hyperimmune serum of whom antibodies are directed against eye tissues for Serocytol “Œil” and nerves, vessels, connective, skin tissues for Serocytol Neurovasculaire.
Results
Forty-two age-related macular degeneration (AMD) patients were included (Table 1). Twenty patients in the treated group (group 1) had the following characteristics: 4 male, 16 female with a mean age of 70.3 years (59-84). Twenty-two patients in the control untreated group (group 2) were composed of 6 male, 16 female with a mean age of 73 years (51-89).
Follow-up was available for a duration of 1456 days (SD 873 days) corresponding to a period of 4 years in groups 1. The control group (group 2) had a mean follow-up period of 1309 days (SD 502 days) corresponding to 3.5 years.
In treated group (group 1) 7 out of 19 (36.8%) were treated with potentially photosensitizing drugs and 12 out of 19 (63.1%) had high blood pressure. In the control group (group 2) 12 out of 22 (54.5%) were taking potentially photosensitizing drugs and 10 out of 22 (45.5%) were hypertensive.
In group 1, 10 patients had an atrophic form of AMD in both eyes, and 10 an exsudative form in at least one eye. In group 2, 12 patients presented an atrophic form of AMD, and 10 an exsudative complication of their AMD.
Fig. 1A, 1B, and 1C
show the AMD evolution by one 75-years old female patient who did not receive Serocytol products. Fig. 2A, 2B, and
2C show the evolution of AMD by one 86-years old female patient treated with Serocytol products.
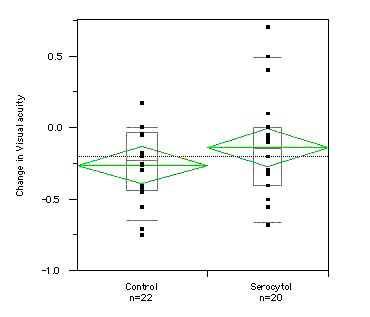
At baseline visit, the mean best corrected VA in group 1 was 0.53 (SD 0.27) and 0.65 (SD 0.27) in group 2. At the end of follow-up: the mean best corrected VA in group 1 and group 2 was identical at 0.39 (SD 0.27 in group 1 and SD 0.31 in group 2). The visual acuity loss was -0.14 (SD 0.38) in the treated group (group 1) and -0.26 (SD 0.25) in the untreated group (group 2).
Fig. 3 shows changes in visual acuity over time in the two groups.
Contrast sensitivity testing at inclusion was available only for group 1 and measured at 1.25 (SD 0.47). At the end of the study, contrast sensitivity was evaluated at 1.06 (SD 0.40) in group 1 and 1.02 (SD 0.48) in group 2.
General anatomic evolution was favorable, corresponding to stability of the RPE atrophy and RPE alterations in 14 patients in group 1 and 5 patients in group 2 and worsen in 6 patients of group 1 and 17 patients of group 2.
No adverse ocular or systemic harmful effect has been observed. Compliance of treatment was perfect as no patient did quit the treatment during follow-up.
Discussion
The retinal pigment epithelium (RPE), situated between the retina and external blood-retinal barrier, play a crucial role to protect the eye fragile tissues from damages [18, 19]. Following numerous reports, AMD is recognized among various etiopathogenies as an inflammatory disease, the earliest hallmark being the accumulation of drusen within the RPE. The insoluble drusen deposits contain a variety of inflammatory molecules from multiple sources including activators of the complement cascade, immune complex, cytokines, chemokines or growth factors. Auto-antibodies to retinal tissue have been detected in sera from patients with AMD, earlier studies have revealed that autoimmune response in the eye might be mediated by RPE cells, since these cells share several similarities with antigen presenting cells and possess a variety of costimulatory molecules [3,
12, 13, 20]. The macrophages dysfunction could also play a role in AMD [17] although much more remain to be elucidated on the interaction between the cellular and molecular events involved in the development of AMD.
The role of antibodies to defend the organism against aggressions by foreign antigens is well known. However antibodies can exert other biological functions such as inhibition or stimulation of the antigenic structures against which they are targeted [8, 22]. It is the cellular immunomodulation, that is emerging as an important field for the treatment of number of chronic disorders [23,
24]. Multiple mechanisms could account for immunomodulatory activities exerted by immunoglobulins and they could act by a direct binding on the target such as T or B cells which results on an increase of T-cell suppressor function and an inhibition of B-cell function, a neutralization of autoimmune antibodies, an interaction with the complement and the cytokine network or an inhibition of Fab-mediated cell death [23,
24].
Intravenous polyclonal immunoglobulins are frequently used to treat chronic inflammatory and autoimmune diseases because of their immunomodulation properties administered at a dose of 400 to 600 mg/kg every 3 to 4 weeks to reach serum IgG level of 500 mg/dl [23]. Animal’s polyclonal hyperimmune antibodies against human thymic lymphocytes induce a profound immunosuppression of cellular immune responses and are used by daily doses from 1.5 to 30 mg/kg for 14 to 28 days to prevent acute organ graft rejection.
In contrast, tissue antisera repeated low-doses have been shown sufficient to improve the outcome of some inflammatory disorders [8,
9, 10, 11].
The daily dose administered by rectal route is of 20 mg of sera proteins. In the clinical practice its take generally about 6 weeks after the beginning of the treatment with these products to observe the therapeutic effect, suggesting a necessary repetitive contact of the active molecules with their targets [25].
Interestingly, this treatment is an easy therapeutical approach known as safe, no long-term side effects have been reported, compared to the conventional intravenous immunoglobulins which have relatively high risk of adverse reactions.
Experimental studies have been performed which demonstrated that equine antibodies, contained in Serocytol®
products and raised against different porcine tissues, bind to corresponding mammalian tissues and consequently induce immunoregulatory effects such as rabbit gastric cells via the (Fab)2 fragment of IgG [26] or human lymphocytes and macrophages [22,
27]. An experiment with suppositories containing 131-I labelled immunoglobulins given by rectal route to volunteers has shown the absorption of Ig fractions [28].
Based on theses immunoregulatory principles, it was decided to assess tissue antisera “Œil” and “Neurovasculaire” in different eye diseases, particularly in AMD.
The authors of the present paper were motivated to initiate a retrospective analysis on the use of equine tissue antisera in the growing hypothesis of immune-mediated processes in AMD well described by Hageman et al in 2001 [3] confirmed by the knowledge of that the
complement factor H (CFH) has been the first major susceptibility gene for AMD.
Hypothesis of sub-cellular mechanism on drusen of tissue antisera:
First of all, our results suggest a good tolerance of equine tissue antisera “Œil” and “Neurovasculaire" used for a mean period of 4 years without any harmful side effects. This good tolerance was furthermore supported by the frequent request of patients to receive the next prescription for the three months cure.
The most important end point to our patients was the slight clinical benefit in the treated group of more than 1 line in best corrected VA. This functional benefit was also supported by a trend in morphological stabilization in the treated group.
Obviously this clinically significant treatment benefit could not be considered statistically significant. The small sample size, and probably the staging of AMD showing a disease process going on for years are the main explanations for the lack of statistical significance.
Due to the retrospective design of the study, both groups were not perfectly matched.
Factors such as differences in initial VA, high blood pressure, photosensitizing drugs intake, and age could interfere with clinical evolution of our AMD patients.
At inclusion, treated group had a lower best corrected VA which could mean that the disease processes was more advanced. This selection bias would mean that the Serocytol® treatment could be more effective in reality then shown in the present study.
A similar conclusion could be drawn due to the higher prevalence of high blood pressure, a well known risk factor for AMD [29], in the treated group.
On the other side, other selection bias could reduce the precision of the study results.
The younger age (2.5 years difference) of the treated group could explain part of the treatment benefit. A similar conclusion could be drawn as less patients were taking photosensitizing drugs, a known risk factors for AMD [12] in the treated group. And finally, lower best corrected VA in the treated group at inclusion could mean that those patients had less vision to loose, leading to an opposite conclusion than the one mentioned above.
An analytical approach through various immunological markers could help us to understand the biological pathways of the immunomodulatory effect of this treatment strategy.
Clinical and basic scientific studies will help to clarify the action by which this therapy may provide some benefits.
A second step would consist in a well-designed randomized and controlled study.
Conclusions
The present study is to our best knowledge the first attempt to evaluate the treatment benefit of an immunomodulatory approach in various forms of AMD. The trend towards clinical benefit and the new physiopathological hypothesis on biogenesis of drusen are both incentive to pursue in this direction.
Competing interests
The authors MMF and MS declare that they have no competing interests.
The authors TW and MM are scientific staff of Serolab.
Authors' contributions
MMF conceived the study, carried out the data acquisition and helped to draft the manuscript. MS participated in the study design, performed the statistical analysis and helped to draft the manuscript. TW participated in the study design and coordination of the data acquisition and helped to draft the manuscript. MM participated in the study design and helped to draft the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Written consent was obtained from both patients for publications of figures 1 and 2.
References
- NM Bressler: Age-related macular degeneration is the leading cause of blindness. Jama 2004, 291:1900-1. [PubMed]
- A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol 2001, 119:1417-36. [PubMed]
- GS Hageman, PJ Luthert, NH Victor Chong, LV Johnson, DH Anderson, RF Mullins: An integrated hypothesis that considers drusen as biomarkers of immune-mediated processes at the RPE-Bruch's membrane interface in aging and age-related macular degeneration. Prog Retin Eye Res 2001, 20:705-32. [PubMed]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J.: Complement factor H polymorphism in age-related macular degeneration. Science. 2005 Apr 15;308(5720):385-9. [PubMed]
- Edwards AO, Ritter R 3rd, Abel KJ, Manning A, Panhuysen C, Farrer LA.: Complement factor H polymorphism and age-related macular degeneration. Science. 2005 Apr 15;308(5720):421-4. [PubMed]
- Haines JL, Hauser MA, Schmidt S, Scott WK, Olson LM, Gallins P, Spencer KL, Kwan SY, Noureddine M, Gilbert JR, Schnetz-Boutaud N, Agarwal A, Postel EA, Pericak-Vance MA.: Complement factor H variant increases the risk of age-related macular degeneration. Science. 2005 Apr 15;308(5720):419-21. [PubMed]
- Hageman GS, Anderson DH, Johnson LV, Hancox LS, Taiber AJ, Hardisty LI, Hageman JL, Stockman HA, Borchardt JD, Gehrs KM, Smith RJ, Silvestri G, Russell SR, Klaver CC, Barbazetto I, Chang S, Yannuzzi LA, Barile GR, Merriam JC, Smith RT, Olsh AK, Bergeron J, Zernant J, Merriam JE, Gold B, Dean M, Allikmets R.: A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci U S A. 2005 May 17;102(20):7227-32. [PubMed]
- F Ginsberg: Comparative clinical evaluation of the activity of Ser-316 suppository in the treatment of lumbar osteoarthrosis. Curr Med Res Opin 1991, 12:413-22. [PubMed]
- C Kempenaers, G Simenon, M Vander Elst, L Fransolet, P Mingard, V de Maertelaer, T Appelboom, J Mendlewicz: Effect of an antidiencephalon immune serum on pain and sleep in primary fibromyalgia. Neuropsychobiology 1994, 30:66-72. [PubMed]
- J Frick, H Joos, G Kunit: Double-blind study with Serocytol "Muqueuse urinaire" and "S.R.E." in patients with urological disorders. Int Urol Nephrol 1985, 17:29-33. [PubMed]
- F Piccione, G Grecomoro, B Pinto, A Sanfilippo: Immune therapy of osteoarthritis: an open assessment of clinical results with heterologous antibodies to articular tissue ('Serocytol'). Pharmatherapeutica 1986, 4:577-84. [PubMed]
- DH Anderson, RF Mullins, GS Hageman, LV Johnson: A role for local inflammation in the formation of drusen in the aging eye. Am J Ophthalmol 2002, 134:411-31. [PubMed]
- PL Penfold, MC Madigan, MC Gillies, JM Provis: Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res 2001, 20:385-414. [PubMed]
- M Mauget-Faysse, M Quaranta, N Francoz, D BenEzra: Incidental retinal phototoxicity associated with ingestion of photosensitizing drugs. Graefes Arch Clin Exp Ophthalmol 2001, 239:501-8. [PubMed]
- E de La Marnierre, B Guigon, M Quaranta, M Mauget-Faysse: [Phototoxic drugs and age-related maculopathy]. J Fr Ophtalmol 2003, 26:596-601. [PubMed]
- JE Lovie-Kitchin: Validity and reliability of visual acuity measurements. Ophthalmic Physiol Opt 1988, 8:363-70. [PubMed]
- DB Elliott, K Sanderson, A Conkey: The reliability of the Pelli-Robson contrast sensitivity chart. Ophthalmic Physiol Opt 1990, 10:21-4. [PubMed]
- F Willermain, L Caspers-Velu, B Nowak, P Stordeur, R Mosselmans, I Salmon, T Velu, C Bruyns: Retinal pigment epithelial cells phagocytosis of T lymphocytes: possible implication in the immune privilege of the eye. Br J Ophthalmol 2002, 86:1417-21. [PubMed]
- MV Kumar, CN Nagineni, MS Chin, JJ Hooks, B Detrick: Innate immunity in the retina: Toll-like receptor (TLR) signaling in human retinal pigment epithelial cells. J Neuroimmunol 2004, 153:7-15. [PubMed]
- PT Johnson, GP Lewis, KC Talaga, MN Brown, PJ Kappel, SK Fisher, DH Anderson, LV Johnson: Drusen-associated degeneration in the retina. Invest Ophthalmol Vis Sci 2003, 44:4481-8. [PubMed]
- EU Irschick, R Sgonc, G Bock, H Wolf, D Fuchs, W Nussbaumer, W Gottinger, HP Huemer: Retinal pigment epithelial phagocytosis and metabolism differ from those of macrophages. Ophthalmic Res 2004, 36:200-10. [PubMed]
- J Clot, M Andary: Anticorps de cheval anti-système réticulo-endothélial (SRE). Spécificité et propriétés vis à vis des cellules du SRE humain. Méd. et Hyg. 1983, 41:2838-2844. [Abstract]
- RP Nelson, Jr., M Ballow: 26. Immunomodulation and immunotherapy: drugs, cytokines, cytokine receptors, and antibodies. J Allergy Clin Immunol 2003, 111:S720-43. [PubMed]
- MD Kazatchkine, SV Kaveri: Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 2001, 345:747-55. [PubMed]
- T Appelboom, M Koutsoukos, M Pierart, J Bentin: Possible anti-anti CD3 (OKT3) activity in RES-1080. Int. J. Immunotherapy 1990, VI:113-118. [Abstract]
- MH Bobo, S Ammor, R Magous, P Mingard, JP Bali: Anti-stomach serum induces contraction of isolated smooth muscle cells from gastric antrum. Immunopharmacology 1993, 26:241-7. [PubMed]
- T Appelboom, N Mairesse, M Pierart, M Koutsoukos, J Bentin: Immunopharmacological properties of RES-1080 in vitro. Int. J. Immunotherapy 1990, VI:105-112. [Abstract]
- G Combépine, J Alexandre, B Van Gansbeke, A Schoutens: Fate of 131I-labeled immunoglobulins given by rectal route to human volunteers. European Immunology Meeting, Zagreb, 1987. [Abstract]
- R Klein, BE Klein, SC Tomany, TY Wong: The relation of retinal microvascular characteristics to age-related eye disease: the Beaver Dam eye study. Am J Ophthalmol 2004, 137:435-44. [PubMed]
Table 1 - Patients and study data
|
|
Control group |
Serocytol group |
| Number of patients |
N = 22 |
N = 20 |
| Sex ratio |
6 men / 16 women |
4 men / 16 women |
| Study eye |
13 right / 9 left |
8 right / 12 left |
| Mean age |
73 years |
70.3 years |
| Photosensitizing agents per patient |
|
|
|
No photosensitizing agent |
9 patients |
13 patients |
|
1 photosensitizing agent |
7 patients |
4 patients |
|
2 photosensitizing agents |
5 patients |
3 patients |
|
3 photosensitizing agents |
1 patient |
no patient |
| AMD category |
12 atrophic / 10 wet |
10 atrophic / 10 wet |
| Mean follow-up period (days) |
1309 ± 502 days |
1456 ± 873 days |
| Baseline visual acuity |
0.65 ± 0.27 |
0.53 ± 0.27 |
| Final visual acuity |
0.39 ± 0.31 |
0.39 ± 0.27 |
| Baseline contrast sensitivity |
Not available |
1.25 ± 0.47 |
| Final contrast sensitivity |
1.02 ± 0.48 |
1.06 ± 0.40 |
|
Morphological changes |
5 stable / 17 worse |
14 stable / 6 worse |
Figures
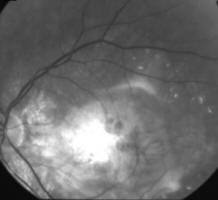
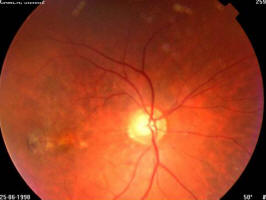
Figure 1A. 75 year old female, March 1999, left eye (controlateral eye): VA = < 20/400, terminal age-related macular degeneration fibroglial scar
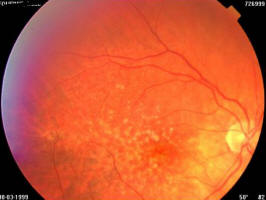
Figure 1B. Same patient, March 1999, right eye (study eye): VA = 0.8, age-related macular degeneration with drusen
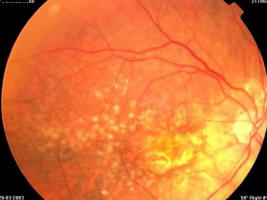
Figure 1C. Same patient, March 2003, right eye (study eye): VA = 0.32, age-related macular degeneration with central retinal pigment epithelium atrophy with central scotoma and a major visual acuity decrease
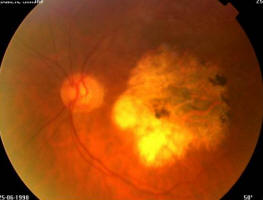
Figure 2A. 86 year old female, June 1998, left eye: VA = 20/200, age-related macular degeneration with perifoveal photocoagulation scar
Figure 2B. Same patient, June 1998, right eye: VA = 20/20, age-related macular degeneration with drusen and some RPE alterations
Figure 2C. Same patient, July 2002, right eye: VA = 20/20, age-related macular degeneration with drusen and some RPE alterations
Figure 3. Changes in visual acuity over time. The mean loss (horizontal line in green) in the control group was -0.26 as compared to a mean loss of -0.14 in the Serocytol treatment group