First Consensus Meeting on Menopause in the East Asian Region
Cardiovascular disease and hormone replacement therapy
Hideo Honjo, Mamoru Urabe, Kazunori Tanaka, Tomohiro Kashiwagi, Tomoharu Okubo, Hiroshi Tsuchiya, Koichi Iwasa, Noriko Kikuchi, Masaaki Fukuoka and Takara Yamamoto
Department of Obstetrics and Gynaecology, Kyoto Prefectural University of Medicine, Kyoto, Japan
Introduction
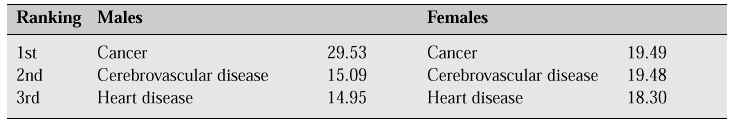
In 1996, the average life span for Japanese women was 83.59 years, the highest worldwide average for 12 years. The second most common cause of death in women is cerebrovascular disease, and the third is heart disease. Both of these conditions are closely associated with arterial sclerosis (Table I).
Table I: Estimated rate of leading causes of death (% of possible causes of death for those born in 1996)[1]
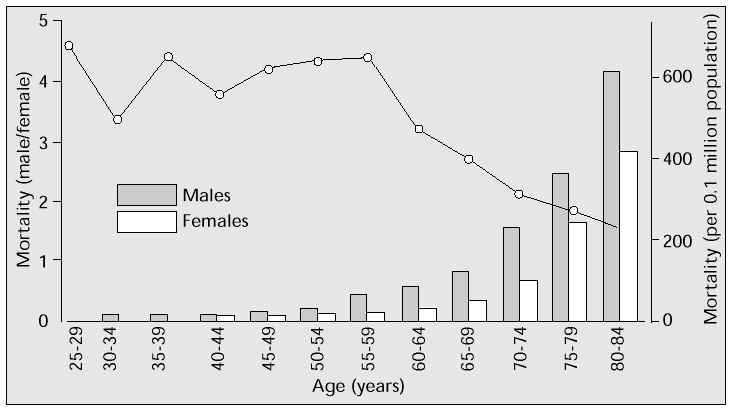
The Population Development Statistics for 1995 by the Japanese Ministry of Health and Welfare [2] show that mortality due to ischaemic heart disease in those aged 25–60 years is approximately four times higher in men than in women. But the male-female gap narrows in the 60-plus age range (i.e. approximately 10 years after the menopause), and after 75 years of age the two groups do not significantly differ (Fig. 1). In other words, postmenopausal women are susceptible to ischaemic heart disease, largely due to the reduction in oestrogen following the menopause.
Fig. 1: Age-related mortality due to ischaemic heart disease in males and females in 1995.
Hyperlipidaemia
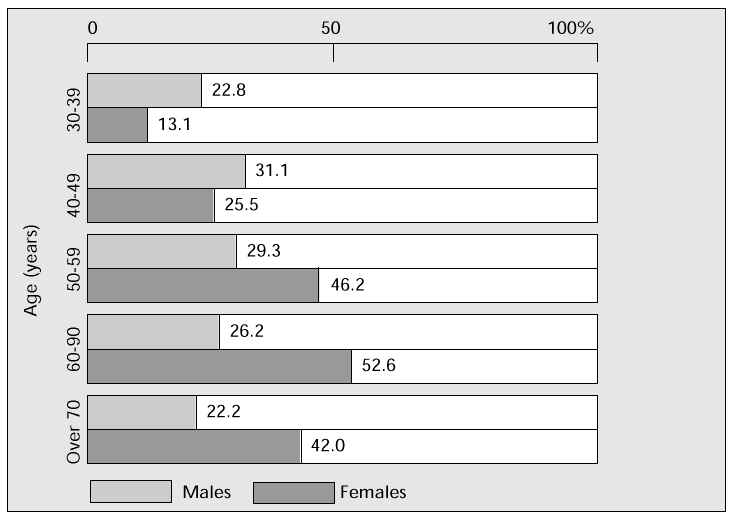
Hyperlipidaemia, the most common cause of arterial sclerosis, is significantly increased in postmenopausal women. Serum total cholesterol (TC) above 220 mg/dl (the critical level, i.e. the level at which, according to the Consensus Conference of the Japanese Arterial Sclerosis Society, winter 1987, treatment of hyperlipidaemia should begin) is seen in 46.2% of women in their fifties, and 52.6% in their sixties. Both figures are considerably higher than those in age-matched men (Fig. 2) [3]. Compared with premenopausal climacteric women, TC in the postmenopausal climacteric group is significantly higher at age 50–52 years [4]. The relationship between menopause, reduction in oestrogen and increase in TC is an important factor in the development of hyperlipidaemia.
Fig. 2: Percentage of men and women with serum TC values above 220 mg/dl.
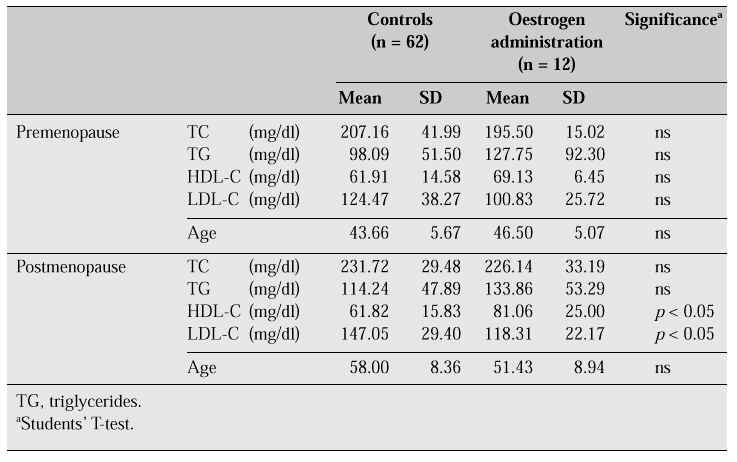
In a comparison between pre- and postmenopausal women, one group on oestrogen replacement therapy (for treatment of climacteric symptoms and osteoporosis) and a control group [5], high-density lipoprotein cholesterol (HDL-C) was found to be raised and low-density lipoprotein cholesterol (LDL-C) reduced in the postmenopausal group on oestrogen replacement therapy (Table II). A significant decrease in TC can generally be demonstrated after oestrogen administration, although this was not demonstrable in this study.
Table II: Serum lipids in pre- and postmenopausal women (effects of extrinsic oestrogen)
The mechanism of action of oestrogen on lipid metabolism
The effects of oestrogen on hepatic triglyceride lipase (HTGL) and lipoprotein lipase (LPL) are compared in Figure 3. Oestrogen replacement therapy in postmenopausal women remarkably suppresses HTGL, while not significantly affecting LPL [6].
Fig. 3: Oestrogen replacement therapy in postmenopausal women significantly suppresses hepatic triglyceride lipase (HTGL), yet has no effect on lipoprotein lipase (LPL).
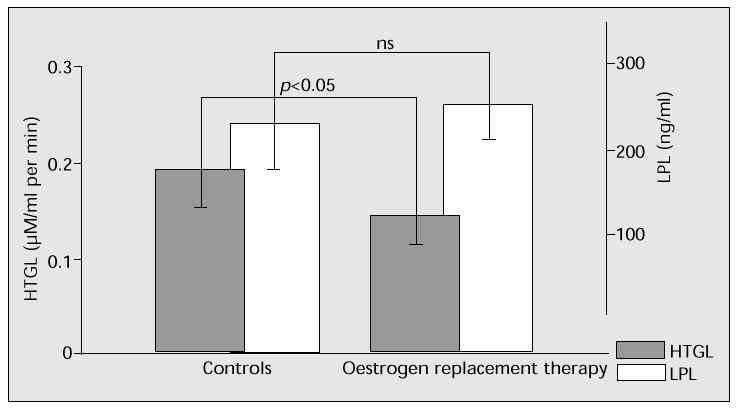
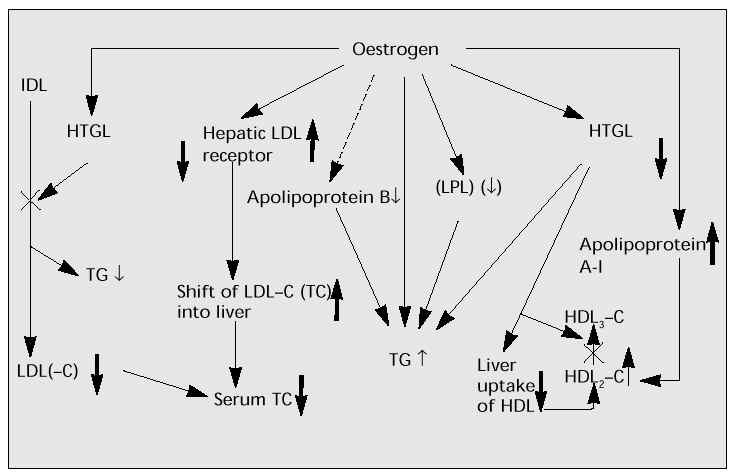
Figure 4 shows the effects of oestrogen on enzymes and lipids [7]. Low- or medium-dose oestrogen reduces TC and raises HDL-C. HTGL accelerates the catabolic conversion of triglyceride (TG)-rich HDL2 to TG-poor HDL3. The suppression and deficit of HTGL may increase HDL2. Also, HTGL induces hepatic uptake of HDL. The suppression and decrease of HTGL may encourage the increase in HDL. Oestrogen may stimulate intestinal and/or hepatic production of apolipoprotein A-I (apo A-I) and increase HDL (the main component of which is Apo A-I). Synergy of these oestrogenic effects may induce the significant increase in HDL-C. HTGL also accelerates the conversion of intermediate-density lipoprotein (IDL) to LDL(-C). The suppression of HTGL by oestrogen results in the reduction of LDL(-C) as well as serum TC. In addition, oestrogen increases hepatic LDL receptors and accelerates the shift of serum LDL-C into the liver, leading to the decrease in serum LDL-C (and TC).
Low- or medium-dose oestrogen has beneficial effects on lipids via the above mechanism. However, high doses may increase serum TG and have an unfavourable effect, causing an increase in hepatic production of TG and inhibition of LPL.
Fig. 4: Beneficial effects of low- to medium-dose oestrogen on enzymes and lipids.
Direct action of oestrogen on arterial sclerosis
Inhibition of hyperlipidaemia by oestrogen prevents and improves arterial sclerosis. Oestrogen also has a direct action on arterial sclerosis by inhibiting oxidation and suppressing the growth or spread of smooth muscle cells.
Inhibition of oxidation
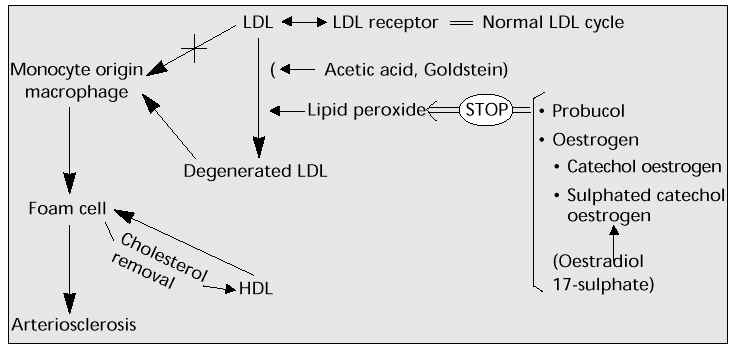
Lipid peroxide degenerates (oxidizes) LDL-C, which is absorbed by the monocyte and turns into foam cells in the vascular subendothelial cavity, leading to atherogenesis in the aorta and eventually arterial sclerosis. Oestrogen, like some other substances, can suppress this oxidation (Fig. 5).
Fig. 5: Lipid peroxidation and sulphated catechol oestrogen (2- or 4-hydroxy oestradiol 17-sulphate).
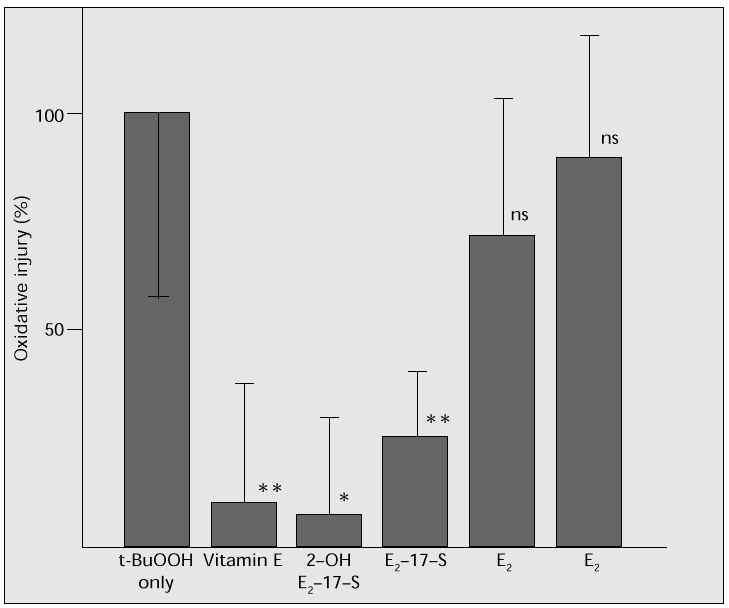
As a model for inhibiting lipid peroxidation, human arterial endothelial cells (Krabow) were cultured, and oxidizing degeneration by t-butyl hydroperoxide was determined using a 51Cr release assay. Oestradiol (17b-oestradiol, E2), oestradiol 17-sulphate (E2-17-S), 2-hydroxy oestradiol 17-sulphate (2-OH E2-17-S) or vitamin E were added to the culture, to compare and study their actions in suppressing oxidation. Addition of 2-OH E2-17-S or vitamin E considerably inhibited oxidation; E2-17-S also showed a significant inhibitory effect (Fig. 6). Oestradiol also has a suppressive effect, even though no significant difference could be demonstrated in this study. This study clearly showed the effective suppression of oxidation by oestrogen, especially E2-17-S and 2-OH E2-17-S. It is thought that these oestrogens may be effective in preventing oxidative stress and antagonizing atherogenesis as well as hypertension.
Fig. 6: Analysis of oxidation of human arterial endothelial cells by t-BuOOH, using a 51 Cr release assay: the addition
of 2-OH E 2 -17-S suppresses oxidation, as does vitamin E. E 2 -17-S also has a suppressive effect.
Means ± SD; *p < 0.01, **p < 0.02.
Oestrogen receptors in vascular smooth muscle cells, and sulphatase
The direct action of oestrogen on the vascular cells involves oestrogen receptors. Some reports have already indicated that vascular smooth muscle cells contain functional oestrogen receptors. We have also studied and been able to confirm the expression of oestrogen receptors in human vascular smooth muscle cells.
Moreover, conjugated oestrone sulphate, the most widely used oestrogen in hormone replacement therapy (HRT), cannot express its oestrogenic effect unless it is changed into active oestrogen through sulphatase in the local tissues. We have also confirmed the expression of sulphatase mRNA in the human vascular smooth muscle cells. These results suggest that human vascular smooth muscle cells contain sulphatase activity which can convert conjugated oestrone sulphate to active oestrone and oestradiol in local cells, leading to direct effects on vascular cells such as oestrogen receptors.
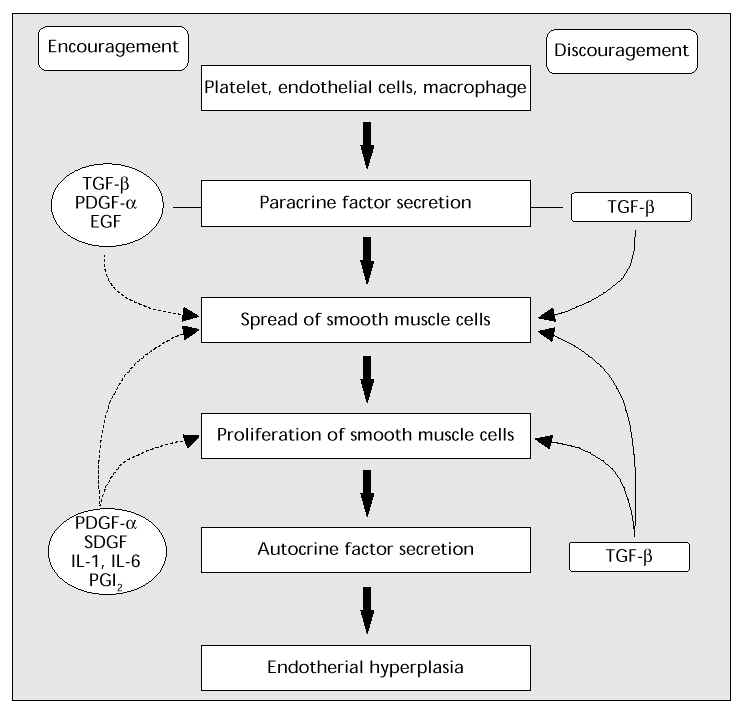
Control of growth and spread of smooth muscle cells
Another important mechanism in arterial sclerosis is the progress of the medial vascular smooth muscle cells (spread into the vascular subendothelial cavity ® proliferation ® arterial sclerosis). It is thought that multiple paracrine or autocrine factors are involved in this process (Fig. 7).
Fig. 7: Control of the growth and spread of smooth muscle cells. TGF- b,transforming growth factor-beta; PDGF- a, platelet-derived growth factor-alpha; EGF, epidermal growth factor; SDGF, smooth muscle cell-derived growth factor; IL-1, interleukin-1; IL-6, interleukin-6; PGI 2 , prostacyclin.
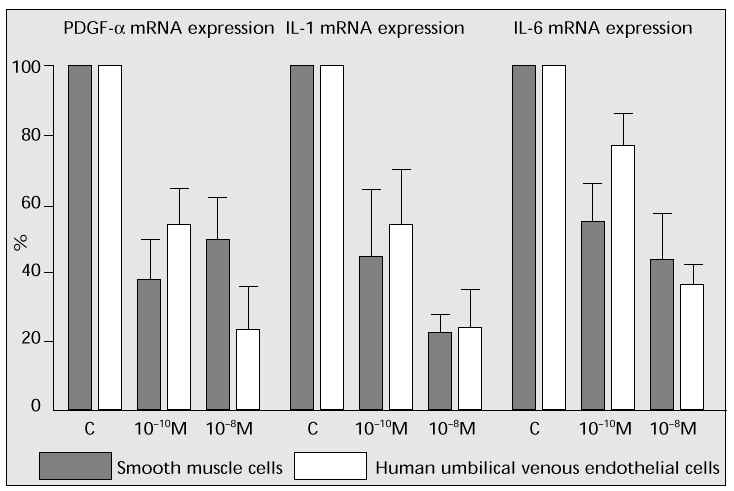
Human vascular smooth muscle cells (derived from aorta, Krabow) or human umbilical venous endothelial cells were cultured to investigate the effect of oestrogen on mRNA expression in each factor. Oestrogen considerably suppressed mRNA expression of platelet-derived growth factor-alpha (PDGF-a), interleukin-1 (IL-1) and interleukin-6 (IL-6), which should accelerate the arterial sclerosis progress (Fig. 8). These outcomes suggest that oestrogen suppresses the progress of arterial sclerosis in both paracrine and autocrine factors.
Fig. 8: The effects of oestrogen on proliferating factors of vascular smooth muscle cells and endo-thelial cells.
Immediate effects of oestrogen on the vessel wall
The immediate effects of oestrogen in women with coronary artery disease have been investigated [8]. Following sublingual administration of 1 mg oestradiol with motor loading 40 min after administration, time until the decrease in ST by 1 mm and time for continuous motor loading were determined and analysed, showing that these time delays are prolonged in the oestradiol treatment group compared with those in the placebo group. An acute/chronic improvement in cerebral blood flow with oestrogen replacement therapy has been also documented [9].
Oestrogen replacement therapy
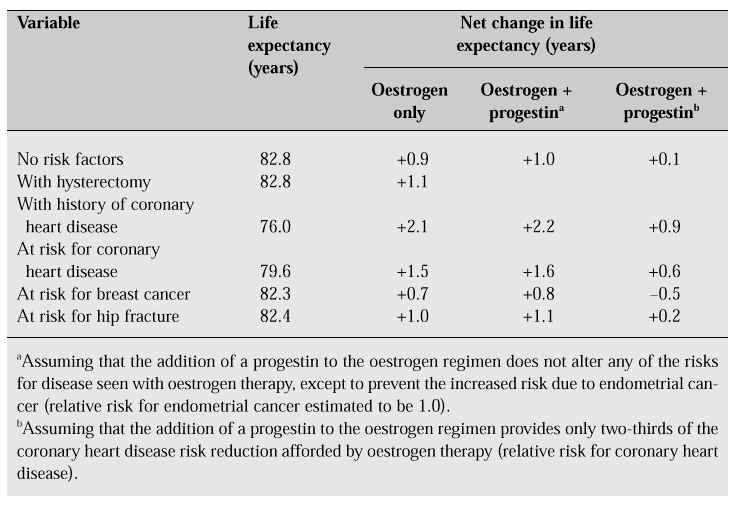
Long-term treatment with oestrogen is thought to improve hyperlipidaemia and arterial sclerosis and to inhibit or improve cardiovascular disease. Oestrogen replacement therapy prolongs the life span by 0.9 years of women with risk factors and by 2.1 years in women with a history of coronary heart disease (Table III) [10].
Table III: Net change in life expectancy of 50-year-old white women treated with long-term hor-mone replacement [10].
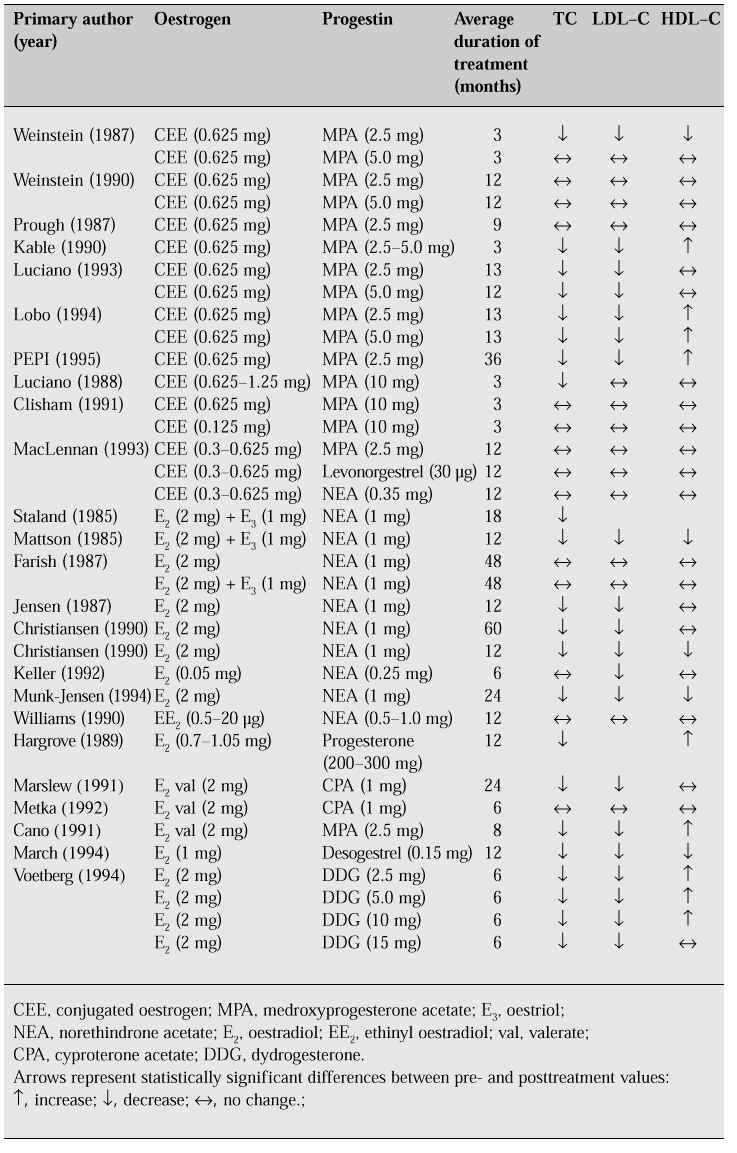
The effect on lipids of HRT, which generally includes progestogen to prevent endometrial cancer, is inconclusive (Table IV).
Table IV: Changes in lipid profiles in women receiving combined continuous oestrogen and proges-tin replacement therapy. (Adapted from [11].)
Oestrogen administered to decrease TC and increase HDL-C can effect an improvement in hyperlipidaemia within 2 months. However, the recently developed 3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (Mevalotin, Bezatol SR) [5] and other antihyperlipidaemic drugs also can improve hyperlipidaemia within 2 weeks. In critical cases where TC exceeds 260 mg/dl, antihyperlipidaemic drugs should be selected as the first choice, or used concomitantly with oestrogen. In the case of high TG levels, large doses of oestrogen should be given with caution. In such cases, Bezatol SR is recommended.
For mild to medium hyperlipidaemia, especially if accompanied by high TC, vasomotor disorders including hot flushes, or osteoporosis, low-dose oestrogen such as conjugated oestrogen (Premarin) is recommended at a dose of 0.625 mg/day or alternatively an oestrogen patch (Estraderm TTS, 2 mg) is also effective, using one patch per administration.
Conclusion
Faced with an increasingly ageing population, the use of HRT in enhancing the quality of life of older women, including its use in the treatment of arterial sclerosis, deserves further study, which may produce even wider applications for its use [12].
References
1. Simple life table in 1996. Japanese Ministry of Health and Welfare, 29 August 1997.
2. Population Development Statistics for 1995. Japanese Ministry of Health and Welfare, 31 August 1996; 404–5.
3. Outline of the 4th Cardiac Disease Basic Survey (Nov 1990) by the Disease Control Division, Health Service Bureau, Koseisho (Ministry of Health and Welfare) (Jan 1993). Mainichi Newspaper, 4 Feb 1993.
4. Honjo H. Clinical results of Mevalotin: study of usefulness against hypercholesterolemia in the climacteric woman. J Am Med Assoc (Japanese version) 1991; 12 (appendix): 26–9.
5. Honjo H, Tanaka K, Urabe M et al. Menopause and hyperlipidemia: pravastatin lowers lipid levels without decreasing endogenous estrogens. Clin Ther 1992; 14: 699–707.
6. Urabe M, Yamamoto T, Kashiwagi T et al. Effect of estrogen replacement therapy on hepatic triglyceride lipase, lipoprotein lipase and lipids including apolipoprotein E in climacteric and elderly women. Endocr J 1996; 43: 737–42.
7. Honjo H, Tanaka K, Kashiwagi T et al. Women of menopausal age and hyperlipidemia: effects of estrogens and an HMG-CoA reductase inhibitor. In: Yamamoto A, ed. Multiple risk factors in cardiovascular disease. Tokyo: Churchill Livingstone 1994; 81–4.
8. Rosano GMC, Sarrel PM, Poole-Wilson PA, Collins P. Beneficial effect of oestrogen on exercise-induced myocardial ischaemia in women with coronary artery disease. Lancet 1993; 342: 133–6.
9. Ohkura T, Teshima Y, Isse K et al. Estrogen increases cerebral and cerebellar blood flows in postmenopausal women. Menopause 1995; 2: 13–8.
10. Grady D, Rubin SM, Petitti DB et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med 1992; 117: 1016–37.
11. Udoff L, Langenberg P, Adashi EY. Combined continuous hormone replacement therapy: a critical review. Obstet Gynecol 1995; 86: 306–16.
12. Honjo H. Climacteric and elderly out-patients’ manual — invitation to queen’s corner. 1st ed., 2nd issue. Kyoto: Kinpodo, 1995.